
The Project Gutenberg EBook of The Evolution of Man, by Ernst Haeckel This eBook is for the use of anyone anywhere in the United States and most other parts of the world at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.org. If you are not located in the United States, you'll have to check the laws of the country where you are located before using this ebook. Title: The Evolution of Man Author: Ernst Haeckel Release Date: August 1, 2003 [EBook #8700] [Most recently updated: April 5, 2020] Language: English Character set encoding: UTF-8 *** START OF THIS PROJECT GUTENBERG EBOOK THE EVOLUTION OF MAN *** Produced by Produced by Sue Asscher and Derek Thompson
Translated from the Fifth (enlarged) Edition by Joseph McCabe
[Issued for the Rationalist Press Association, Limited]
WATTS & CO.
17 Johnson’s Court, Fleet Street, London, E.C.
1912

Acrania: animals without skull (cranium).
Anthropogeny: the evolution (genesis) of man (anthropos).
Anthropology: the science of man.
Archi-: (in compounds) the first or typical—as, archi-cytula,
archi-gastrula, etc.
Biogeny: the science of the genesis of life (bios).
Blast-: (in compounds) pertaining to the early embryo (blastos = a
bud); hence:—
Blastoderm: skin (derma) or enclosing layer of the embryo.
Blastosphere: the embryo in the hollow sphere stage.
Blastula: same as preceding.
Epiblast: the outer layer of the embryo (ectoderm).
Hypoblast: the inner layer of the embryo (endoderm).
Branchial: pertaining to the gills (branchia).
Caryo-: (in compounds) pertaining to the nucleus (caryon); hence:—
Caryokineses: the movement of the nucleus.
Caryolysis: dissolution of the nucleus.
Caryoplasm: the matter of the nucleus.
Centrolecithal: see under Lecith-.
Chordaria and Chordonia: animals with a dorsal chord or back-bone.
Cœlom or Cœloma: the body-cavity in the embryo; hence:—
Cœlenterata: animals without a body-cavity.
Cœlomaria: animals with a body-cavity.
Cœlomation: formation of the body-cavity.
Cyto-: (in compounds) pertaining to the cell (cytos); hence:—
Cytoblast: the nucleus of the cell.
Cytodes: cell-like bodies, imperfect cells.
Cytoplasm: the matter of the body of the cell.
Cytosoma: the body (soma) of the cell.
Cryptorchism: abnormal retention of the testicles in the body.
Deutoplasm: see Plasm.
Dualism: the belief in the existence of two entirely distinct
principles (such as matter and spirit).
Dysteleology: the science of those features in organisms which refute
the “design-argument”.
Ectoderm: the outer (ekto) layer of the embryo.
Entoderm: the inner (ento) layer of the embryo.
Epiderm: the outer layer of the skin.
Epigenesis: the theory of gradual development of organs in the embryo.
Epiphysis: the third or central eye in the early vertebrates.
Episoma: see Soma.
Epithelia: tissues covering the surface of parts of the body (such as
the mouth, etc.)
Gonads: the sexual glands.
Gonochorism: separation of the male and female sexes.
Gonotomes: sections of the sexual glands.
Gynecomast: a male with the breasts (masta) of a woman (gyne).
Hepatic: pertaining to the liver (hepar).
Holoblastic: embryos in which the animal and vegetal cells divide
equally (holon = whole).
Hypermastism: the possession of more than the normal breasts (masta).
Hypobranchial: underneath (hypo) the gills.
Hypophysis: sensitive-offshoot from the brain in the vertebrate.
Hyposoma: see Soma.
Lecith-: pertaining to the yelk (lecithus); hence:—
Centrolecithal: eggs with the yelk in the centre.
Lecithoma: the yelk-sac.
Telolecithal: eggs with the yelk at one end.
Meroblastic: cleaving in part (meron) only.
Meta-: (in compounds) the “after” or secondary stage; hence:—
Metagaster: the secondary or permanent gut (gaster).
Metaplasm: secondary or differentiated plasm.
Metastoma: the secondary or permanent mouth (stoma).
Metazoa: the higher or later animals, made up of many cells.
Metovum: the mature or advanced ovum.
Metamera: the segments into which the embryo breaks up.
Metamerism: the segmentation of the embryo.
Monera: the most primitive of the unicellular organisms.
Monism: belief in the fundamental unity of all things.
Morphology: the science of organic forms (generally equivalent to
anatomy).
Myotomes: segments into which the muscles break up.
Nephra: the kidneys; hence:—
Nephridia: the rudimentary kidney-organs.
Nephrotomes: the segments of the developing kidneys.
Ontogeny: the science of the development of the individual (generally
equivalent to embryology).
Perigenesis: the genesis of the movements in the vital particles.
Phagocytes: cells that absorb food (phagein = to eat).
Phylogeny: the science of the evolution of species (phyla).
Planocytes: cells that move about (planein).
Plasm: the colloid or jelly-like matter of which organisms are
composed; hence:—
Caryoplasm: the matter of the nucleus (caryon).
Cytoplasm: the matter of the body of the cell.
Deutoplasm: secondary or differentiated plasm.
Metaplasm: secondary or differentiated plasm.
Protoplasm: primitive or undifferentiated plasm.
Plasson: the simplest form of plasm.
Plastidules: small particles of plasm.
Polyspermism: the penetration of more than one sperm-cell into the ovum.
Pro- or Prot: (in compounds) the earlier form (opposed to Meta); hence:—
Prochorion: the first form of the chorion.
Progaster: the first or primitive stomach.
Pronephridia: the earlier form of the kidneys.
Prorenal: the earlier form of the kidneys.
Prostoma: the first or primitive mouth.
Protists: the earliest or unicellular organisms.
Provertebræ: the earliest phase of the vertebræ.
Protophyta: the primitive or unicellular plants.
Protoplasm: undifferentiated plasm.
Protozoa: the primitive or unicellular animals.
Renal: pertaining to the kidneys (renes).
Scatulation: packing or boxing-up (scatula = a box).
Sclerotomes: segments into which the primitive skeleton falls.
Soma: the body; hence:—
Cytosoma: the body of the cell (cytos).
Episoma: the upper or back-half of the embryonic body.
Somites: segments of the embryonic body.
Hyposoma: the under or belly-half of the embryonic body.
Teleology: the belief in design and purpose (telos) in nature.
Telolecithal: see Lecith-.
Umbilical: pertaining to the navel (umbilicus).
Vitelline: pertaining to the yelk (vitellus).
[BY JOSEPH MCCABE]
The work which we now place within the reach of every reader of the English tongue is one of the finest productions of its distinguished author. The first edition appeared in 1874. At that time the conviction of man’s natural evolution was even less advanced in Germany than in England, and the work raised a storm of controversy. Theologians—forgetting the commonest facts of our individual development—spoke with the most profound disdain of the theory that a Luther or a Goethe could be the outcome of development from a tiny speck of protoplasm. The work, one of the most distinguished of them said, was “a fleck of shame on the escutcheon of Germany.” To-day its conclusion is accepted by influential clerics, such as the Dean of Westminster, and by almost every biologist and anthropologist of distinction in Europe. Evolution is not a laboriously reached conclusion, but a guiding truth, in biological literature to-day.
There was ample evidence to substantiate the conclusion even in the first edition of the book. But fresh facts have come to light in each decade, always enforcing the general truth of man’s evolution, and at times making clearer the line of development. Professor Haeckel embodied these in successive editions of his work. In the fifth edition, of which this is a translation, reference will be found to the very latest facts bearing on the evolution of man, such as the discovery of the remarkable effect of mixing human blood with that of the anthropoid ape. Moreover, the ample series of illustrations has been considerably improved and enlarged; there is no scientific work published, at a price remotely approaching that of the present edition, with so abundant and excellent a supply of illustrations. When it was issued in Germany, a few years ago, a distinguished biologist wrote in the Frankfurter Zeitung that it would secure immortality for its author, the most notable critic of the idea of immortality. And the Daily Telegraph reviewer described the English version as a “handsome edition of Haeckel’s monumental work,” and “an issue worthy of the subject and the author.”
The influence of such a work, one of the most constructive that Haeckel has ever written, should extend to more than the few hundred readers who are able to purchase the expensive volumes of the original issue. Few pages in the story of science are more arresting and generally instructive than this great picture of “mankind in the making.” The horizon of the mind is healthily expanded as we follow the search-light of science down the vast avenues of past time, and gaze on the uncouth forms that enter into, or illustrate, the line of our ancestry. And if the imagination recoils from the strange and remote figures that are lit up by our search-light, and hesitates to accept them as ancestral forms, science draws aside another veil and reveals another picture to us. It shows us that each of us passes, in our embryonic development, through a series of forms hardly less uncouth and unfamiliar. Nay, it traces a parallel between the two series of forms. It shows us man beginning his existence, in the ovary of the female infant, as a minute and simple speck of jelly-like plasm. It shows us (from analogy) the fertilised ovum breaking into a cluster of cohering cells, and folding and curving, until the limb-less, head-less, long-tailed fœtus looks like a worm-shaped body. It then points out how gill-slits and corresponding blood-vessels appear, as in a lowly fish, and the fin-like extremities bud out and grow into limbs, and so on; until, after a very clear ape-stage, the definite human form emerges from the series of transformations.
It is with this embryological evidence for our evolution that the present volume is concerned. There are illustrations in the work that will make the point clear at a glance. Possibly too clear; for the simplicity of the idea and the eagerness to apply it at every point have carried many, who borrow hastily from Haeckel, out of their scientific depth. Haeckel has never shared their errors, nor encouraged their superficiality. He insists from the outset that a complete parallel could not possibly be expected. Embryonic life itself is subject to evolution. Though there is a general and substantial law—as most of our English and American authorities admit—that the embryonic series of forms recalls the ancestral series of forms, the parallel is blurred throughout and often distorted. It is not the obvious resemblance of the embryos of different animals, and their general similarity to our extinct ancestors in this or that organ, on which we must rest our case. A careful study must be made of the various stages through which all embryos pass, and an effort made to prove their real identity and therefore genealogical relation.
This is a task of great subtlety and delicacy. Many scientists have worked at it together with Professor Haeckel—I need only name our own Professor Balfour and Professor Ray Lankester—and the scheme is fairly complete. But the general reader must not expect that even so clear a writer as Haeckel can describe these intricate processes without demanding his very careful attention. Most of the chapters in the present volume (and the second volume will be less difficult) are easily intelligible to all; but there are points at which the line of argument is necessarily subtle and complex. In the hope that most readers will be induced to master even these more difficult chapters, I will give an outline of the characteristic argument of the work. Haeckel’s distinctive services in regard to man’s evolution have been: (1) The construction of a complete ancestral tree, though, of course, some of the stages in it are purely conjectural, and not final; (2) The tracing of the remarkable reproduction of ancestral forms in the embryonic development of the individual. Naturally, he has not worked alone in either department. The second volume of this work will embody the first of these two achievements; the present one is mainly concerned with the latter. It will be useful for the reader to have a synopsis of the argument and an explanation of some of the chief terms invented or employed by the author.
The main theme of the work is that, in the course of their embryonic development, all animals, including man, pass roughly and rapidly through a series of forms which represents the succession of their ancestors in the past. After a severe and extensive study of embryonic phenomena, Haeckel has drawn up a “law” (in the ordinary scientific sense) to this effect, and has called it “the biogenetic law,” or the chief law relating to the evolution (genesis) of life (bios). This law is widely and increasingly accepted by embryologists and zoologists. It is enough to quote a recent declaration of the great American zoologist, President D. Starr Jordan: “It is, of course, true that the life-history of the individual is an epitome of the life-history of the race”; while a distinguished German zoologist (Sarasin) has described it as being of the same use to the biologist as spectrum analysis is to the astronomer.
But the reproduction of ancestral forms in the course of the embryonic development is by no means always clear, or even always present. Many of the embryonic phases do not recall ancestral stages at all. They may have done so originally, but we must remember that the embryonic life itself has been subject to adaptive changes for millions of years. All this is clearly explained by Professor Haeckel. For the moment, I would impress on the reader the vital importance of fixing the distinction from the start. He must thoroughly familiarise himself with the meaning of five terms. Biogeny is the development of life in general (both in the individual and the species), or the sciences describing it. Ontogeny is the development (embryonic and post-embryonic) of the individual (on), or the science describing it. Phylogeny is the development of the race or stem (phulon), or the science describing it. Roughly, ontogeny may be taken to mean embryology, and phylogeny what we generally call evolution. Further, the embryonic phenomena sometimes reproduce ancestral forms, and they are then called palingenetic (from palin = again): sometimes they do not recall ancestral forms, but are later modifications due to adaptation, and they are then called cenogenetic (from kenos = new or foreign). These terms are now widely used, but the reader of Haeckel must understand them thoroughly.
The first five chapters are an easy account of the history of embryology and evolution. The sixth and seventh give an equally clear account of the sexual elements and the process of conception. But some of the succeeding chapters must deal with embryonic processes so unfamiliar, and pursue them through so wide a range of animals in a brief space, that, in spite of the 200 illustrations, they will offer difficulty to many a reader. As our aim is to secure, not a superficial acquiescence in conclusions, but a fair comprehension of the truths of science, we have retained these chapters. However, I will give a brief and clear outline of the argument, so that the reader with little leisure may realise their value.
When the animal ovum (egg-cell) has been fertilised, it divides and subdivides until we have a cluster of cohering cells, externally not unlike a raspberry or mulberry. This is the morula (= mulberry) stage. The cluster becomes hollow, or filled with fluid in the centre, all the cells rising to the surface. This is the blastula (hollow ball) stage. One half of the cluster then bends or folds in upon the other, as one might do with a thin indiarubber ball, and we get a vase-shaped body with hollow interior (the first stomach, or “primitive gut”), an open mouth (the first or “primitive mouth”), and a wall composed of two layers of cells (two “germinal layers”). This is the gastrula (stomach) stage, and the process of its formation is called gastrulation. A glance at the illustration (Fig. 29) will make this perfectly clear.
So much for the embryonic process in itself. The application to evolution has been a long and laborious task. Briefly, it was necessary to show that all the multicellular animals passed through these three stages, so that our biogenetic law would enable us to recognise them as reminiscences of ancestral forms. This is the work of Chapters VIII and IX. The difficulty can be realised in this way: As we reach the higher animals the ovum has to take up a large quantity of yelk, on which it may feed in developing. Think of the bird’s “egg.” The effect of this was to flatten the germ (the morula and blastula) from the first, and so give, at first sight, a totally different complexion to what it has in the lowest animals. When we pass the reptile and bird stage, the large yelk almost disappears (the germ now being supplied with blood by the mother), but the germ has been permanently altered in shape, and there are now a number of new embryonic processes (membranes, blood-vessel connections, etc.). Thus it was no light task to trace the identity of this process of gastrulation in all the animals. It has been done, however; and with this introduction the reader will be able to follow the proof. The conclusion is important. If all animals pass through the curious gastrula stage, it must be because they all had a common ancestor of that nature. To this conjectural ancestor (it lived before the period of fossilisation begins) Haeckel gives the name of the Gastræa, and in the second volume we shall see a number of living animals of this type (“gastræads”).
The line of argument is the same in the next chapter. After laborious and careful research (though this stage is not generally admitted in the same sense as the previous one), a fourth common stage was discovered, and given the name of the Cœlomula. The blastula had one layer of cells, the blastoderm (derma = skin): the gastrula two layers, the ectoderm (“outer skin”) and entoderm (“inner skin”). Now a third layer (mesoderm = middle skin) is formed, by the growth inwards of two pouches or folds of the skin. The pouches blend together, and form a single cavity (the body cavity, or cœlom), and its two walls are two fresh “germinal layers.” Again, the identity of the process has to be proved in all the higher classes of animals, and when this is done we have another ancestral stage, the Cœlomæa.
The remaining task is to build up the complex frame of the higher animals—always showing the identity of the process (on which the evolutionary argument depends) in enormously different conditions of embryonic life—out of the four “germinal layers.” Chapter IX prepares us for the work by giving us a very clear account of the essential structure of the back-boned (vertebrate) animal, and the probable common ancestor of all the vertebrates (a small fish of the lancelet type). Chapters XI–XIV then carry out the construction step by step. The work is now simpler, in the sense that we leave all the invertebrate animals out of account; but there are so many organs to be fashioned out of the four simple layers that the reader must proceed carefully. In the second volume each of these organs will be dealt with separately, and the parallel will be worked out between its embryonic and its phylogenetic (evolutionary) development. The general reader may wait for this for a full understanding. But in the meantime the wonderful story of the construction of all our organs in the course of a few weeks (the human frame is perfectly formed, though less than two inches in length, by the twelfth week) from so simple a material is full of interest. It would be useless to attempt to summarise the process. The four chapters are themselves but a summary of it, and the eighty fine illustrations of the process will make it sufficiently clear. The last chapter carries the story on to the point where man at last parts company with the anthropoid ape, and gives a full account of the membranes or wrappers that enfold him in the womb, and the connection with the mother.
In conclusion, I would urge the reader to consult, at his free library perhaps, the complete edition of this work, when he has read the present abbreviated edition. Much of the text has had to be condensed in order to bring out the work at our popular price, and the beautiful plates of the complete edition have had to be omitted. The reader will find it an immense assistance if he can consult the library edition.
JOSEPH MCCABE
Cricklewood, March, 1906.
| Unicellular animals (Protozoa) | ||
| 1. Unnucleated | Bacteria Protamæbæ | Monera |
| 2. Nucleated | a. Rhizopoda | Amœbina Radiolaria |
| b. Infusoria | Flagellata Ciliata | |
| 3. Cell-colonies | Catallacta Blastæada | |
| Unicellular animals (Protozoa) | |||
| I Cœlenterata, or Zoophytes. Animals without body-cavity, blood, or anus. |
a. Gastræads | Gastremaria Cyemaria | |
| b. Sponges | Protospongiæ Metaspongiæ | ||
| c. Cnidaria (stinging animals) |
Hydrozoa Polyps Medusæ | ||
| d. Platodes (flat-worms) | Platodaria Turbullaria Trematoda Cestoda | ||
|
II Cœlomaria or Bilaterals. Animals with body-cavity and anus, and generally blood. |
a. Vermalia (worm-like) |
Rotatoria Strongylaria Prosopygia Frontonia | |
| b. Molluscs | Cochlides Conchades Teuthodes | ||
| c. Articulates | Annelida Crustacea Tracheata | ||
| d. Echinoderms | Monorchonia Pentorchonia | ||
| e. Tunicates | Copelata Ascidiæ Thalidiæ |
||
| f. Vertebrates | I. Acrania-Lancelet (without skull) II. Craniota (with skull) a. Cyclostomes (“round-mouthed”) | ||
| b. Fishes | Selachii Ganoids Teleosts Dipneusts | ||
| c. Amphibia d. Reptiles e. Birds | |||
| f. Mammal | Monotremes Marsupials Placentals: Rodents Edentates Ungulates Cetacea Sirenia Insectivora Cheiroptera Carnassia Primates | ||
(This classification is given for the purpose of explaining Haeckel’s use of terms in this volume. The general reader should bear in mind that it differs very considerably from more recent schemes of classification. He should compare the scheme framed by Professor E. Ray Lankester.)
The field of natural phenomena into which I would introduce my readers in the following chapters has a quite peculiar place in the broad realm of scientific inquiry. There is no object of investigation that touches man more closely, and the knowledge of which should be more acceptable to him, than his own frame. But among all the various branches of the natural history of mankind, or anthropology, the story of his development by natural means must excite the most lively interest. It gives us the key of the great world-riddles at which the human mind has been working for thousands of years. The problem of the nature of man, or the question of man’s place in nature, and the cognate inquiries as to the past, the earliest history, the present situation, and the future of humanity—all these most important questions are directly and intimately connected with that branch of study which we call the science of the evolution of man, or, in one word, “Anthropogeny” (the genesis of man). Yet it is an astonishing fact that the science of the evolution of man does not even yet form part of the scheme of general education. In fact, educated people even in our day are for the most part quite ignorant of the important truths and remarkable phenomena which anthropogeny teaches us.
As an illustration of this curious state of things, it may be pointed out that most of what are considered to be “educated” people do not know that every human being is developed from an egg, or ovum, and that this egg is one simple cell, like any other plant or animal egg. They are equally ignorant that in the course of the development of this tiny, round egg-cell there is first formed a body that is totally different from the human frame, and has not the remotest resemblance to it. Most of them have never seen such a human embryo in the earlier period of its development, and do not know that it is quite indistinguishable from other animal embryos. At first the embryo is no more than a round cluster of cells, then it becomes a simple hollow sphere, the wall of which is composed of a layer of cells. Later it approaches very closely, at one period, to the anatomic structure of the lancelet, afterwards to that of a fish, and again to the typical build of the amphibia and mammals. As it continues to develop, a form appears which is like those we find at the lowest stage of mammal-life (such as the duck-bills), then a form that resembles the marsupials, and only at a late stage a form that has a resemblance to the ape; until at last the definite human form emerges and closes the series of transformations. These suggestive facts are, as I said, still almost unknown to the general public—so completely unknown that, if one casually mentions them, they are called in question or denied outright as fairy-tales. Everybody knows that the butterfly emerges from the pupa, and the pupa from a quite different thing called a larva, and the larva from the butterfly’s egg. But few besides medical men are aware that man, in the course of his individual formation, passes through a series of transformations which are not less surprising and wonderful than the familiar metamorphoses of the butterfly.
The mere description of these remarkable changes through which man passes during his embryonic life should arouse considerable interest. But the mind will experience a far keener satisfaction when we trace these curious facts to their causes, and when we learn to behold in them natural phenomena which are of the highest importance throughout the whole field of human knowledge. They throw light first of all on the “natural history of creation,” then on psychology, or “the science of the soul,” and through this on the whole of philosophy. And as the general results of every branch of inquiry are summed up in philosophy, all the sciences come in turn to be touched and influenced more or less by the study of the evolution of man.
But when I say that I propose to present here the most important features of these phenomena and trace them to their causes, I take the term, and I interpret my task, in a very much wider sense than is usual. The lectures which have been delivered on this subject in the universities during the last half-century are almost exclusively adapted to medical men. Certainly, the medical man has the greatest interest in studying the origin of the human body, with which he is daily occupied. But I must not give here this special description of the embryonic processes such as it has hitherto been given, as most of my readers have not studied anatomy, and are not likely to be entrusted with the care of the adult organism. I must content myself with giving some parts of the subject only in general outline, and must not enter upon all the marvellous, but very intricate and not easily described, details that are found in the story of the development of the human frame. To understand these fully a knowledge of anatomy is needed. I will endeavour to be as plain as possible in dealing with this branch of science. Indeed, a sufficient general idea of the course of the embryonic development of man can be obtained without going too closely into the anatomic details. I trust we may be able to arouse the same interest in this delicate field of inquiry as has been excited already in other branches of science; though we shall meet more obstacles here than elsewhere.
The story of the evolution of man, as it has hitherto been expounded to medical students, has usually been confined to embryology—more correctly, ontogeny—or the science of the development of the individual human organism. But this is really only the first part of our task, the first half of the story of the evolution of man in that wider sense in which we understand it here. We must add as the second half—as another and not less important and interesting branch of the science of the evolution of the human stem—phylogeny: this may be described as the science of the evolution of the various animal forms from which the human organism has been developed in the course of countless ages. Everybody now knows of the great scientific activity that was occasioned by the publication of Darwin’s Origin of Species in 1859. The chief direct consequence of this publication was to provoke a fresh inquiry into the origin of the human race, and this has proved beyond question our gradual evolution from the lower species. We give the name of “Phylogeny” to the science which describes this ascent of man from the lower ranks of the animal world. The chief source that it draws upon for facts is “Ontogeny,” or embryology, the science of the development of the individual organism. Moreover, it derives a good deal of support from paleontology, or the science of fossil remains, and even more from comparative anatomy, or morphology.
These two branches of our science—on the one side ontogeny or embryology, and on the other phylogeny, or the science of race-evolution—are most vitally connected. The one cannot be understood without the other. It is only when the two branches fully co-operate and supplement each other that “Biogeny” (or the science of the genesis of life in the widest sense) attains to the rank of a philosophic science. The connection between them is not external and superficial, but profound, intrinsic, and causal. This is a discovery made by recent research, and it is most clearly and correctly expressed in the comprehensive law which I have called “the fundamental law of organic evolution,” or “the fundamental law of biogeny.” This general law, to which we shall find ourselves constantly recurring, and on the recognition of which depends one’s whole insight into the story of evolution, may be briefly expressed in the phrase: “The history of the fœtus is a recapitulation of the history of the race”; or, in other words, “Ontogeny is a recapitulation of phylogeny.” It may be more fully stated as follows: The series of forms through which the individual organism passes during its development from the ovum to the complete bodily structure is a brief, condensed repetition of the long series of forms which the animal ancestors of the said organism, or the ancestral forms of the species, have passed through from the earliest period of organic life down to the present day.
The causal character of the relation which connects embryology with stem-history is due to the action of heredity and adaptation. When we have rightly understood these, and recognised their great importance in the formation of organisms, we can go a step further and say: Phylogenesis is the mechanical cause of ontogenesis.[1] In other words, the development of the stem, or race, is, in accordance with the laws of heredity and adaptation, the cause of all the changes which appear in a condensed form in the evolution of the fœtus.
[1] The term “genesis,” which occurs throughout, means, of course, “birth” or origin. From this we get: Biogeny = the origin of life (bios); Anthropogeny = the origin of man (anthropos); Ontogeny = the origin of the individual (on); Phylogeny = the origin of the species (phulon); and so on. In each case the term may refer to the process itself, or to the science describing the process.—Translator.
The chain of manifold animal forms which represent the ancestry of each higher organism, or even of man, according to the theory of descent, always form a connected whole. We may designate this uninterrupted series of forms with the letters of the alphabet: A, B, C, D, E, etc., to Z. In apparent contradiction to what I have said, the story of the development of the individual, or the ontogeny of most organisms, only offers to the observer a part of these forms; so that the defective series of embryonic forms would run: A, B, D, F, H, K, M, etc.; or, in other cases, B, D, H, L, M, N, etc. Here, then, as a rule, several of the evolutionary forms of the original series have fallen out. Moreover, we often find—to continue with our illustration from the alphabet—one or other of the original letters of the ancestral series represented by corresponding letters from a different alphabet. Thus, instead of the Roman B and D, we often have the Greek Β and Δ. In this case the text of the biogenetic law has been corrupted, just as it had been abbreviated in the preceding case. But, in spite of all this, the series of ancestral forms remains the same, and we are in a position to discover its original complexion.
In reality, there is always a certain parallel between the two evolutionary series. But it is obscured from the fact that in the embryonic succession much is wanting that certainly existed in the earlier ancestral succession. If the parallel of the two series were complete, and if this great fundamental law affirming the causal connection between ontogeny and phylogeny in the proper sense of the word were directly demonstrable, we should only have to determine, by means of the microscope and the dissecting knife, the series of forms through which the fertilised ovum passes in its development; we should then have before us a complete picture of the remarkable series of forms which our animal ancestors have successively assumed from the dawn of organic life down to the appearance of man. But such a repetition of the ancestral history by the individual in its embryonic life is very rarely complete. We do not often find our full alphabet. In most cases the correspondence is very imperfect, being greatly distorted and falsified by causes which we will consider later. We are thus, for the most part, unable to determine in detail, from the study of its embryology, all the different shapes which an organism’s ancestors have assumed; we usually—and especially in the case of the human fœtus—encounter many gaps. It is true that we can fill up most of these gaps satisfactorily with the help of comparative anatomy, but we cannot do so from direct embryological observation. Hence it is important that we find a large number of lower animal forms to be still represented in the course of man’s embryonic development. In these cases we may draw our conclusions with the utmost security as to the nature of the ancestral form from the features of the form which the embryo momentarily assumes.
To give a few examples, we can infer from the fact that the human ovum is a simple cell that the first ancestor of our species was a tiny unicellular being, something like the amœba. In the same way, we know, from the fact that the human fœtus consists, at the first, of two simple cell-layers (the gastrula), that the gastræa, a form with two such layers, was certainly in the line of our ancestry. A later human embryonic form (the chordula) points just as clearly to a worm-like ancestor (the prochordonia), the nearest living relation of which is found among the actual ascidiæ. To this succeeds a most important embryonic stage (acrania), in which our headless fœtus presents, in the main, the structure of the lancelet. But we can only indirectly and approximately, with the aid of comparative anatomy and ontogeny, conjecture what lower forms enter into the chain of our ancestry between the gastræa and the chordula, and between this and the lancelet. In the course of the historical development many intermediate structures have gradually fallen out, which must certainly have been represented in our ancestry. But, in spite of these many, and sometimes very appreciable, gaps, there is no contradiction between the two successions. In fact, it is the chief purpose of this work to prove the real harmony and the original parallelism of the two. I hope to show, on a substantial basis of facts, that we can draw most important conclusions as to our genealogical tree from the actual and easily-demonstrable series of embryonic changes. We shall then be in a position to form a general idea of the wealth of animal forms which have figured in the direct line of our ancestry in the lengthy history of organic life.
In this evolutionary appreciation of the facts of embryology we must, of course, take particular care to distinguish sharply and clearly between the primitive, palingenetic (or ancestral) evolutionary processes and those due to cenogenesis.[2] By palingenetic processes, or embryonic recapitulations, we understand all those phenomena in the development of the individual which are transmitted from one generation to another by heredity, and which, on that account, allow us to draw direct inferences as to corresponding structures in the development of the species. On the other hand, we give the name of cenogenetic processes, or embryonic variations, to all those phenomena in the fœtal development that cannot be traced to inheritance from earlier species, but are due to the adaptation of the fœtus, or the infant-form, to certain conditions of its embryonic development. These cenogenetic phenomena are foreign or later additions; they allow us to draw no direct inference whatever as to corresponding processes in our ancestral history, but rather hinder us from doing so.
[2] Palingenesis = new birth, or re-incarnation (palin = again, genesis or genea = development); hence its application to the phenomena which are recapitulated by heredity from earlier ancestral forms. Cenogenesis = foreign or negligible development (kenos and genea); hence, those phenomena which come later in the story of life to disturb the inherited structure, by a fresh adaptation to environment.—Translator.
This careful discrimination between the primary or palingenetic processes and the secondary or cenogenetic is of great importance for the purposes of the scientific history of a species, which has to draw conclusions from the available facts of embryology, comparative anatomy, and paleontology, as to the processes in the formation of the species in the remote past. It is of the same importance to the student of evolution as the careful distinction between genuine and spurious texts in the works of an ancient writer, or the purging of the real text from interpolations and alterations, is for the student of philology. It is true that this distinction has not yet been fully appreciated by many scientists. For my part, I regard it as the first condition for forming any just idea of the evolutionary process, and I believe that we must, in accordance with it, divide embryology into two sections—palingenesis, or the science of recapitulated forms; and cenogenesis, or the science of supervening structures.
To give at once a few examples from the science of man’s origin in illustration of this important distinction, I may instance the following processes in the embryology of man, and of all the higher vertebrates, as palingenetic: the formation of the two primary germinal layers and of the primitive gut, the undivided structure of the dorsal nerve-tube, the appearance of a simple axial rod between the medullary tube and the gut, the temporary formation of the gill-clefts and arches, the primitive kidneys, and so on.[3] All these, and many other important structures, have clearly been transmitted by a steady heredity from the early ancestors of the mammal, and are, therefore, direct indications of the presence of similar structures in the history of the stem. On the other hand, this is certainly not the case with the following embryonic forms, which we must describe as cenogenetic processes: the formation of the yelk-sac, the allantois, the placenta, the amnion, the serolemma, and the chorion—or, generally speaking, the various fœtal membranes and the corresponding changes in the blood vessels. Further instances are: the dual structure of the heart cavity, the temporary division of the plates of the primitive vertebræ and lateral plates, the secondary closing of the ventral and intestinal walls, the formation of the navel, and so on. All these and many other phenomena are certainly not traceable to similar structures in any earlier and completely-developed ancestral form, but have arisen simply by adaptation to the peculiar conditions of embryonic life (within the fœtal membranes). In view of these facts, we may now give the following more precise expression to our chief law of biogeny: The evolution of the fœtus (or ontogenesis) is a condensed and abbreviated recapitulation of the evolution of the stem (or phylogenesis); and this recapitulation is the more complete in proportion as the original development (or palingenesis) is preserved by a constant heredity; on the other hand, it becomes less complete in proportion as a varying adaptation to new conditions increases the disturbing factors in the development (or cenogenesis).
[3] All these, and the following structures, will be fully described in later chapters.—Translator.
The cenogenetic alterations or distortions of the original palingenetic course of development take the form, as a rule, of a gradual displacement of the phenomena, which is slowly effected by adaptation to the changed conditions of embryonic existence during the course of thousands of years. This displacement may take place as regards either the position or the time of a phenomenon.
The great importance and strict regularity of the time-variations in embryology have been carefully studied recently by Ernest Mehnert, in his Biomechanik (Jena, 1898). He contends that our biogenetic law has not been impaired by the attacks of its opponents, and goes on to say: “Scarcely any piece of knowledge has contributed so much to the advance of embryology as this; its formulation is one of the most signal services to general biology. It was not until this law passed into the flesh and blood of investigators, and they had accustomed themselves to see a reminiscence of ancestral history in embryonic structures, that we witnessed the great progress which embryological research has made in the last two decades.” The best proof of the correctness of this opinion is that now the most fruitful work is done in all branches of embryology with the aid of this biogenetic law, and that it enables students to attain every year thousands of brilliant results that they would never have reached without it.
It is only when one appreciates the cenogenetic processes in relation to the palingenetic, and when one takes careful account of the changes which the latter may suffer from the former, that the radical importance of the biogenetic law is recognised, and it is felt to be the most illuminating principle in the science of evolution. In this task of discrimination it is the silver thread in relation to which we can arrange all the phenomena of this realm of marvels—the “Ariadne thread,” which alone enables us to find our way through this labyrinth of forms. Hence the brothers Sarasin, the zoologists, could say with perfect justice, in their study of the evolution of the Ichthyophis, that “the great biogenetic law is just as important for the zoologist in tracing long-extinct processes as spectrum analyses is for the astronomer.”
Even at an earlier period, when a correct acquaintance with the evolution of the human and animal frame was only just being obtained—and that is scarcely eighty years ago!—the greatest astonishment was felt at the remarkable similarity observed between the embryonic forms, or stages of fœtal development, in very different animals; attention was called even then to their close resemblance to certain fully-developed animal forms belonging to some of the lower groups. The older scientists (Oken, Treviranus, and others) knew perfectly well that these lower forms in a sense illustrated and fixed, in the hierarchy of the animal world, a temporary stage in the evolution of higher forms. The famous anatomist Meckel spoke in 1821 of a “similarity between the development of the embryo and the series of animals.” Baer raised the question in 1828 how far, within the vertebrate type, the embryonic forms of the higher animals assume the permanent shapes of members of lower groups. But it was impossible fully to understand and appreciate this remarkable resemblance at that time. We owe our capacity to do this to the theory of descent; it is this that puts in their true light the action of heredity on the one hand and adaptation on the other. It explains to us the vital importance of their constant reciprocal action in the production of organic forms. Darwin was the first to teach us the great part that was played in this by the ceaseless struggle for existence between living things, and to show how, under the influence of this (by natural selection), new species were produced and maintained solely by the interaction of heredity and adaptation. It was thus Darwinism that first opened our eyes to a true comprehension of the supremely important relations between the two parts of the science of organic evolution—Ontogeny and Phylogeny.
Heredity and adaptation are, in fact, the two constructive physiological functions of living things; unless we understand these properly we can make no headway in the study of evolution. Hence, until the time of Darwin no one had a clear idea of the real nature and causes of embryonic development. It was impossible to explain the curious series of forms through which the human embryo passed; it was quite unintelligible why this strange succession of animal-like forms appeared in the series at all. It had previously been generally assumed that the man was found complete in all his parts in the ovum, and that the development consisted only in an unfolding of the various parts, a simple process of growth. This is by no means the case. On the contrary, the whole process of the development of the individual presents to the observer a connected succession of different animal-forms; and these forms display a great variety of external and internal structure. But why each individual human being should pass through this series of forms in the course of his embryonic development it was quite impossible to say until Lamarck and Darwin established the theory of descent. Through this theory we have at last detected the real causes, the efficient causes, of the individual development; we have learned that these mechanical causes suffice of themselves to effect the formation of the organism, and that there is no need of the final causes which were formerly assumed. It is true that in the academic philosophies of our time these final causes still figure very prominently; in the new philosophy of nature we can entirely replace them by efficient causes. We shall see, in the course of our inquiry, how the most wonderful and hitherto insoluble enigmas in the human and animal frame have proved amenable to a mechanical explanation, by causes acting without prevision, through Darwin’s reform of the science of evolution. We have everywhere been able to substitute unconscious causes, acting from necessity, for conscious, purposive causes.[4]
[4] The monistic or mechanical philosophy of nature holds that only unconscious, necessary, efficient causes are at work in the whole field of nature, in organic life as well as in inorganic changes. On the other hand, the dualist or vitalist philosophy of nature affirms that unconscious forces are only at work in the inorganic world, and that we find conscious, purposive, or final causes in organic nature.
If the new science of evolution had done no more than this, every thoughtful man would have to admit that it had accomplished an immense advance in knowledge. It means that in the whole of philosophy that tendency which we call monistic, in opposition to the dualistic, which has hitherto prevailed, must be accepted.[5] At this point the science of human evolution has a direct and profound bearing on the foundations of philosophy. Modern anthropology has, by its astounding discoveries during the second half of the nineteenth century, compelled us to take a completely monistic view of life. Our bodily structure and its life, our embryonic development and our evolution as a species, teach us that the same laws of nature rule in the life of man as in the rest of the universe. For this reason, if for no others, it is desirable, nay, indispensable, that every man who wishes to form a serious and philosophic view of life, and, above all, the expert philosopher, should acquaint himself with the chief facts of this branch of science.
[5] Monism is neither purely materialistic nor purely spiritualistic, but a reconciliation of these two principles, since it regards the whole of nature as one, and sees only efficient causes at work in it. Dualism, on the contrary, holds that nature and spirit, matter and force, the world and God, inorganic and organic nature, are separate and independent existences. Cf. The Riddle of the Universe, chap. xii.
The facts of embryology have so great and obvious a significance in this connection that even in recent years dualist and teleological philosophers have tried to rid themselves of them by simply denying them. This was done, for instance, as regards the fact that man is developed from an egg, and that this egg or ovum is a simple cell, as in the case of other animals. When I had explained this pregnant fact and its significance in my History of Creation, it was described in many of the theological journals as a dishonest invention of my own. The fact that the embryos of man and the dog are, at a certain stage of their development, almost indistinguishable was also denied. When we examine the human embryo in the third or fourth week of its development, we find it to be quite different in shape and structure from the full-grown human being, but almost identical with that of the ape, the dog, the rabbit, and other mammals, at the same stage of ontogeny. We find a bean-shaped body of very simple construction, with a tail below and a pair of fins at the sides, something like those of a fish, but very different from the limbs of man and the mammals. Nearly the whole front half of the body is taken up by a shapeless head without face, at the sides of which we find gill-clefts and arches as in the fish. At this stage of its development the human embryo does not differ in any essential detail from that of the ape, dog, horse, ox, etc., at a corresponding period. This important fact can easily be verified at any moment by a comparison of the embryos of man, the dog, rabbit, etc. Nevertheless, the theologians and dualist philosophers pronounced it to be a materialistic invention; even scientists, to whom the facts should be known, have sought to deny them.
There could not be a clearer proof of the profound importance of these embryological facts in favour of the monistic philosophy than is afforded by these efforts of its opponents to get rid of them by silence or denial. The truth is that these facts are most inconvenient for them, and are quite irreconcilable with their views. We must be all the more pressing on our side to put them in their proper light. I fully agree with Huxley when he says, in his Man’s Place in Nature: “Though these facts are ignored by several well-known popular leaders, they are easy to prove, and are accepted by all scientific men; on the other hand, their importance is so great that those who have once mastered them will, in my opinion, find few other biological discoveries to astonish them.”
We shall make it our chief task to study the evolution of man’s bodily frame and its various organs in their external form and internal structures. But I may observe at once that this is accompanied step by step with a study of the evolution of their functions. These two branches of inquiry are inseparably united in the whole of anthropology, just as in zoology (of which the former is only a section) or general biology. Everywhere the peculiar form of the organism and its structures, internal and external, is directly related to the special physiological functions which the organism or organ has to execute. This intimate connection of structure and function, or of the instrument and the work done by it, is seen in the science of evolution and all its parts. Hence the story of the evolution of structures, which is our immediate concern, is also the history of the development of functions; and this holds good of the human organism as of any other.
At the same time, I must admit that our knowledge of the evolution of functions is very far from being as complete as our acquaintance with the evolution of structures. One might say, in fact, that the whole science of evolution has almost confined itself to the study of structures; the evolution of functions hardly exists even in name. That is the fault of the physiologists, who have as yet concerned themselves very little about evolution. It is only in recent times that physiologists like W. Engelmann, W. Preyer, M. Verworn, and a few others, have attacked the evolution of functions.
It will be the task of some future physiologist to engage in the study of the evolution of functions with the same zeal and success as has been done for the evolution of structures in morphogeny (the science of the genesis of forms). Let me illustrate the close connection of the two by a couple of examples. The heart in the human embryo has at first a very simple construction, such as we find in permanent form among the ascidiæ and other low organisms; with this is associated a very simple system of circulation of the blood. Now, when we find that with the full-grown heart there comes a totally different and much more intricate circulation, our inquiry into the development of the heart becomes at once, not only an anatomical, but also a physiological, study. Thus it is clear that the ontogeny of the heart can only be understood in the light of its phylogeny (or development in the past), both as regards function and structure. The same holds true of all the other organs and their functions. For instance, the science of the evolution of the alimentary canal, the lungs, or the sexual organs, gives us at the same time, through the exact comparative investigation of structure-development, most important information with regard to the evolution of the functions of these organs.
This significant connection is very clearly seen in the evolution of the nervous system. This system is in the economy of the human body the medium of sensation, will, and even thought, the highest of the psychic functions; in a word, of all the various functions which constitute the proper object of psychology. Modern anatomy and physiology have proved that these psychic functions are immediately dependent on the fine structure and the composition of the central nervous system, or the internal texture of the brain and spinal cord. In these we find the elaborate cell-machinery, of which the psychic or soul-life is the physiological function. It is so intricate that most men still look upon the mind as something supernatural that cannot be explained on mechanical principles.
But embryological research into the gradual appearance and the formation of this important system of organs yields the most astounding and significant results. The first sketch of a central nervous system in the human embryo presents the same very simple type as in the other vertebrates. A spinal tube is formed in the external skin of the back, and from this first comes a simple spinal cord without brain, such as we find to be the permanent psychic organ in the lowest type of vertebrate, the amphioxus. Not until a later stage is a brain formed at the anterior end of this cord, and then it is a brain of the most rudimentary kind, such as we find permanently among the lower fishes. This simple brain develops step by step, successively assuming forms which correspond to those of the amphibia, the reptiles, the duck-bills, and the lemurs. Only in the last stage does it reach the highly organised form which distinguishes the apes from the other vertebrates, and which attains its full development in man.
Comparative physiology discovers a precisely similar growth. The function of the brain, the psychic activity, rises step by step with the advancing development of its structure.
Thus we are enabled, by this story of the evolution of the nervous system, to understand at length the natural development of the human mind and its gradual unfolding. It is only with the aid of embryology that we can grasp how these highest and most striking faculties of the animal organism have been historically evolved. In other words, a knowledge of the evolution of the spinal cord and brain in the human embryo leads us directly to a comprehension of the historic development (or phylogeny) of the human mind, that highest of all faculties, which we regard as something so marvellous and supernatural in the adult man. This is certainly one of the greatest and most pregnant results of evolutionary science. Happily our embryological knowledge of man’s central nervous system is now so adequate, and agrees so thoroughly with the complementary results of comparative anatomy and physiology, that we are thus enabled to obtain a clear insight into one of the highest problems of philosophy, the phylogeny of the soul, or the ancestral history of the mind of man. Our chief support in this comes from the embryological study of it, or the ontogeny of the soul. This important section of psychology owes its origin especially to W. Preyer, in his interesting works, such as The Mind of the Child. The Biography of a Baby (1900), of Milicent Washburn Shinn, also deserves mention. [See also Preyer’s Mental Development in the Child (translation), and Sully’s Studies of Childhood and Children’s Ways.]
In this way we follow the only path along which we may hope to reach the solution of this difficult problem.
Thirty-six years have now elapsed since, in my General Morphology, I established phylogeny as an independent science and showed its intimate causal connection with ontogeny; thirty years have passed since I gave in my gastræa-theory the proof of the justice of this, and completed it with the theory of germinal layers. When we look back on this period we may ask, What has been accomplished during it by the fundamental law of biogeny? If we are impartial, we must reply that it has proved its fertility in hundreds of sound results, and that by its aid we have acquired a vast fund of knowledge which we should never have obtained without it.
There has been no dearth of attacks—often violent attacks—on my conception of an intimate causal connection between ontogenesis and phylogenesis; but no other satisfactory explanation of these important phenomena has yet been offered to us. I say this especially with regard to Wilhelm His’s theory of a “mechanical evolution,” which questions the truth of phylogeny generally, and would explain the complicated embryonic processes without going beyond by simple physical changes—such as the bending and folding of leaves by electricity, the origin of cavities through unequal strain of the tissues, the formation of processes by uneven growth, and so on. But the fact is that these embryological phenomena themselves demand explanation in turn, and this can only be found, as a rule, in the corresponding changes in the long ancestral series, or in the physiological functions of heredity and adaptation.
It is in many ways useful, on entering upon the study of any science, to cast a glance at its historical development. The saying that “everything is best understood in its growth” has a distinct application to science. While we follow its gradual development we get a clearer insight into its aims and objects. Moreover, we shall see that the present condition of the science of human evolution, with all its characteristics, can only be rightly understood when we examine its historical growth. This task will, however, not detain us long. The study of man’s evolution is one of the latest branches of natural science, whether you consider the embryological or the phylogenetic section of it.
Apart from the few germs of our science which we find in classical antiquity, and which we shall notice presently, we may say that it takes its definite rise, as a science, in the year 1759, when one of the greatest German scientists, Caspar Friedrich Wolff, published his Theoria generationis. That was the foundation-stone of the science of animal embryology. It was not until fifty years later, in 1809, that Jean Lamarck published his Philosophie Zoologique—the first effort to provide a base for the theory of evolution; and it was another half-century before Darwin’s work appeared (in 1859), which we may regard as the first scientific attainment of this aim. But before we go further into this solid establishment of evolution, we must cast a brief glance at that famous philosopher and scientist of antiquity, who stood alone in this, as in many other branches of science, for more than 2000 years: the “father of Natural History,” Aristotle.
The extant scientific works of Aristotle deal with many different sides of biological research; the most comprehensive of them is his famous History of Animals. But not less interesting is the smaller work, On the Generation of Animals (Peri zoon geneseos). This work treats especially of embryonic development, and it is of great interest as being the earliest of its kind and the only one that has come down to us in any completeness from classical antiquity.
Aristotle studied embryological questions in various classes of animals, and among the lower groups he learned many most remarkable facts which we only rediscovered between 1830 and 1860. It is certain, for instance, that he was acquainted with the very peculiar mode of propagation of the cuttlefishes, or cephalopods, in which a yelk-sac hangs out of the mouth of the fœtus. He knew, also, that embryos come from the eggs of the bee even when they have not been fertilised. This “parthenogenesis” (or virgin-birth) of the bees has only been established in our time by the distinguished zoologist of Munich, Siebold. He discovered that male bees come from the unfertilised, and female bees only from the fertilised, eggs. Aristotle further states that some kinds of fishes (of the genus serranus) are hermaphrodites, each individual having both male and female organs and being able to fertilise itself; this, also, has been recently confirmed. He knew that the embryo of many fishes of the shark family is attached to the mother’s body by a sort of placenta, or nutritive organ very rich in blood; apart from these, such an arrangement is only found among the higher mammals and man. This placenta of the shark was looked upon as legendary for a long time, until Johannes Müller proved it to be a fact in 1839. Thus a number of remarkable discoveries were found in Aristotle’s embryological work, proving a very good acquaintance of the great scientist—possibly helped by his predecessors—with the facts of ontogeny, and a great advance upon succeeding generations in this respect.
In the case of most of these discoveries he did not merely describe the fact, but added a number of observations on its significance. Some of these theoretical remarks are of particular interest, because they show a correct appreciation of the nature of the embryonic processes. He conceives the development of the individual as a new formation, in the course of which the various parts of the body take shape successively. When the human or animal frame is developed in the mother’s body, or separately in an egg, the heart—which he regards as the starting-point and centre of the organism—must appear first. Once the heart is formed the other organs arise, the internal ones before the external, the upper (those above the diaphragm) before the lower (or those beneath the diaphragm). The brain is formed at an early stage, and the eyes grow out of it. These observations are quite correct. And, if we try to form some idea from these data of Aristotle’s general conception of the embryonic process, we find a dim prevision of the theory which Wolff showed 2000 years afterwards to be the correct view. It is significant, for instance, that Aristotle denied the eternity of the individual in any respect. He said that the species or genus, the group of similar individuals, might be eternal, but the individual itself is temporary. It comes into being in the act of procreation, and passes away at death.
During the 2000 years after Aristotle no progress whatever was made in general zoology, or in embryology in particular. People were content to read, copy, translate, and comment on Aristotle. Scarcely a single independent effort at research was made in the whole of the period. During the Middle Ages the spread of strong religious beliefs put formidable obstacles in the way of independent scientific investigation. There was no question of resuming the advance of biology. Even when human anatomy began to stir itself once more in the sixteenth century, and independent research was resumed into the structure of the developed body, anatomists did not dare to extend their inquiries to the unformed body, the embryo, and its development. There were many reasons for the prevailing horror of such studies. It is natural enough, when we remember that a Bull of Boniface VIII excommunicated every man who ventured to dissect a human corpse. If the dissection of a developed body were a crime to be thus punished, how much more dreadful must it have seemed to deal with the embryonic body still enclosed in the womb, which the Creator himself had decently veiled from the curiosity of the scientist! The Christian Church, then putting many thousands to death for unbelief, had a shrewd presentiment of the menace that science contained against its authority. It was powerful enough to see that its rival did not grow too quickly.
It was not until the Reformation broke the power of the Church, and a refreshing breath of the spirit dissolved the icy chains that bound science, that anatomy and embryology, and all the other branches of research, could begin to advance once more. However, embryology lagged far behind anatomy. The first works on embryology appear at the beginning of the sixteenth century. The Italian anatomist, Fabricius ab Aquapendente, a professor at Padua, opened the advance. In his two books (De formato fœtu, 1600, and De formatione fœtus, 1604) he published the older illustrations and descriptions of the embryos of man and other mammals, and of the hen. Similar imperfect illustrations were given by Spigelius (De formato fœtu, 1631), and by Needham (1667) and his more famous compatriot, Harvey (1652), who discovered the circulation of the blood in the animal body and formulated the important principle, Omne vivum ex vivo (all life comes from pre-existing life). The Dutch scientist, Swammerdam, published in his Bible of Nature the earliest observations on the embryology of the frog and the division of its egg-yelk. But the most important embryological studies in the sixteenth century were those of the famous Italian, Marcello Malpighi, of Bologna, who led the way both in zoology and botany. His treatises, De formatione pulli and De ovo incubato (1687), contain the first consistent description of the development of the chick in the fertilised egg.
Here I ought to say a word about the important part played by the chick in the growth of our science. The development of the chick, like that of the young of all other birds, agrees in all its main features with that of the other chief vertebrates, and even of man. The three highest classes of vertebrates—mammals, birds, and reptiles (lizards, serpents, tortoises, etc.)—have from the beginning of their embryonic development so striking a resemblance in all the chief points of structure, and especially in their first forms, that for a long time it is impossible to distinguish between them. We have known now for some time that we need only examine the embryo of a bird, which is the easiest to get at, in order to learn the typical mode of development of a mammal (and therefore of man). As soon as scientists began to study the human embryo, or the mammal-embryo generally, in its earlier stages about the middle and end of the seventeenth century, this important fact was very quickly discovered. It is both theoretically and practically of great value. As regards the theory of evolution, we can draw the most weighty inferences from this similarity between the embryos of widely different classes of animals. But for the practical purposes of embryological research the discovery is invaluable, because we can fill up the gaps in our imperfect knowledge of the embryology of the mammals from the more thoroughly studied embryology of the bird. Hens’ eggs are easily to be had in any quantity, and the development of the chick may be followed step by step in artificial incubation. The development of the mammal is much more difficult to follow, because here the embryo is not detached and enclosed in a large egg, but the tiny ovum remains in the womb until the growth is completed. Hence, it is very difficult to keep up sustained observation of the various stages in any great extent, quite apart from such extrinsic considerations as the cost, the technical difficulties, and many other obstacles which we encounter when we would make an extensive study of the fertilised mammal. The chicken has, therefore, always been the chief object of study in this connection. The excellent incubators we now have enable us to observe it in any quantity and at any stage of development, and so follow the whole course of its formation step by step.
By the end of the seventeenth century Malpighi had advanced as far as it was possible to do with the imperfect microscope of his time in the embryological study of the chick. Further progress was arrested until the instrument and the technical methods should be improved. The vertebrate embryos are so small and delicate in their earlier stages that you cannot go very far into the study of them without a good microscope and other technical aid. But this substantial improvement of the microscope and the other apparatus did not take place until the beginning of the nineteenth century.
Embryology made scarcely any advance in the first half of the eighteenth century, when the systematic natural history of plants and animals received so great an impulse through the publication of Linné’s famous Systema Naturæ. Not until 1759 did the genius arise who was to give it an entirely new character, Caspar Friedrich Wolff. Until then embryology had been occupied almost exclusively in unfortunate and misleading efforts to build up theories on the imperfect empirical material then available.
The theory which then prevailed, and remained in favour throughout nearly the whole of the eighteenth century, was commonly called at that time “the evolution theory”; it is better to describe it as “the preformation theory.”[6] Its chief point is this: There is no new formation of structures in the embryonic development of any organism, animal or plant, or even of man; there is only a growth, or unfolding, of parts which have been constructed or pre-formed from all eternity, though on a very small scale and closely packed together. Hence, every living germ contains all the organs and parts of the body, in the form and arrangement they will present later, already within it, and thus the whole embryological process is merely an evolution in the literal sense of the word, or an unfolding, of parts that were pre-formed and folded up in it. So, for instance, we find in the hen’s egg not merely a simple cell, that divides and subdivides and forms germinal layers, and at last, after all kinds of variation and cleavage and reconstruction, brings forth the body of the chick; but there is in every egg from the first a complete chicken, with all its parts made and neatly packed. These parts are so small or so transparent that the microscope cannot detect them. In the hatching, these parts merely grow larger, and spread out in the normal way.
[6] This theory is usually known as the “evolution theory” in Germany, in contradistinction to the “epigenesis theory.” But as it is the latter that is called the “evolution theory” in England, France, and Italy, and “evolution” and “epigenesis” are taken to be synonymous, it seems better to call the first the “pre-formation theory.”
When this theory is consistently developed it becomes a “scatulation theory.”[7] According to its teaching, there was made in the beginning one couple or one individual of each species of animal or plant; but this one individual contained the germs of all the other individuals of the same species who should ever come to life. As the age of the earth was generally believed at that time to be fixed by the Bible at 5000 or 6000 years, it seemed possible to calculate how many individuals of each species had lived in the period, and so had been packed inside the first being that was created. The theory was consistently extended to man, and it was affirmed that our common parent Eve had had stored in her ovary the germs of all the children of men.
[7] “Packing theory” would be the literal translation. Scatula is the Latin for a case or box.—Translator.
The theory at first took the form of a belief that it was the females who were thus encased in the first being. One couple of each species was created, but the female contained in her ovary all the future individuals of the species, of either sex. However, this had to be altered when the Dutch microscopist, Leeuwenhoek, discovered the male spermatozoa in 1690, and showed that an immense number of these extremely fine and mobile thread-like beings exist in the male sperm (this will be explained in Chapter VII). This astonishing discovery was further advanced when it was proved that these living bodies, swimming about in the seminal fluid, were real animalcules, and, in fact, were the pre-formed germs of the future generation. When the male and female procreative elements came together at conception, these thread-like spermatozoa (“seed-animals”) were supposed to penetrate into the fertile body of the ovum and begin to develop there, as the plant seed does in the fruitful earth. Hence, every spermatozoon was regarded as a homunculus, a tiny complete man; all the parts were believed to be pre-formed in it, and merely grew larger when it reached its proper medium in the female ovum. This theory, also, was consistently developed in the sense that in each of these thread-like bodies the whole of its posterity was supposed to be present in the minutest form. Adam’s sexual glands were thought to have contained the germs of the whole of humanity.
This “theory of male scatulation” found itself at once in keen opposition to the prevailing “female” theory. The two rival theories at once opened a very lively campaign, and the physiologists of the eighteenth century were divided into two great camps—the Animalculists and the Ovulists—which fought vigorously. The animalculists held that the spermatozoa were the true germs, and appealed to the lively movements and the structure of these bodies. The opposing party of the Ovulists, who clung to the older “evolution theory,” affirmed that the ovum is the real germ, and that the spermatozoa merely stimulate it at conception to begin its growth; all the future generations are stored in the ovum. This view was held by the great majority of the biologists of the eighteenth century, in spite of the fact that Wolff proved it in 1759 to be without foundation. It owed its prestige chiefly to the circumstance that the most weighty authorities in the biology and philosophy of the day decided in favour of it, especially Haller, Bonnet, and Leibnitz.
Albrecht Haller, professor at Göttingen, who is often called the father of physiology, was a man of wide and varied learning, but he does not occupy a very high position in regard to insight into natural phenomena. He made a vigorous defence of the “evolutionary theory” in his famous work, Elementa physiologiae, affirming: “There is no such thing as formation (nulla est epigenesis). No part of the animal frame is made before another; all were made together.” He thus denied that there was any evolution in the proper sense of the word, and even went so far as to say that the beard existed in the new-born child and the antlers in the hornless fawn; all the parts were there in advance, and were merely hidden from the eye of man for the time being. Haller even calculated the number of human beings that God must have created on the sixth day and stored away in Eve’s ovary. He put the number at 200,000 millions, assuming the age of the world to be 6000 years, the average age of a human being to be thirty years, and the population of the world at that time to be 1000 millions. And the famous Haller maintained all this nonsense, in spite of its ridiculous consequences, even after Wolff had discovered the real course of embryonic development and established it by direct observation!
Among the philosophers of the time the distinguished Leibnitz was the chief defender of the “preformation theory,” and by his authority and literary prestige won many adherents to it. Supported by his system of monads, according to which body and soul are united in inseparable association and by their union form the individual, or the “monad,” Leibnitz consistently extended the “scatulation theory” to the soul, and held that this was no more evolved than the body. He says, for instance, in his Théodicée: “I mean that these souls, which one day are to be the souls of men, are present in the seed, like those of other species; in such wise that they existed in our ancestors as far back as Adam, or from the beginning of the world, in the forms of organised bodies.”
The theory seemed to receive considerable support from the observations of one of its most zealous supporters, Bonnet. In 1745 he discovered, in the plant-louse, a case of parthenogenesis, or virgin-birth, an interesting form of reproduction that has lately been found by Siebold and others among various classes of the articulata, especially crustacea and insects. Among these and other animals of certain lower species the female may reproduce for several generations without having been fertilised by the male. These ova that do not need fertilisation are called “false ova,” pseudova or spores. Bonnet saw that a female plant-louse, which he had kept in cloistral isolation, and rigidly removed from contact with males, had on the eleventh day (after forming a new skin for the fourth time) a living daughter, and during the next twenty days ninety-four other daughters; and that all of them went on to reproduce in the same way without any contact with males. It seemed as if this furnished an irrefutable proof of the truth of the scatulation theory, as it was held by the Ovulists; it is not surprising to find that the theory then secured general acceptance.
This was the condition of things when suddenly, in 1759, Caspar Friedrich Wolff appeared, and dealt a fatal blow at the whole preformation theory with his new theory of epigenesis. Wolff, the son of a Berlin tailor, was born in 1733, and went through his scientific and medical studies, first at Berlin under the famous anatomist Meckel, and afterwards at Halle. Here he secured his doctorate in his twenty-sixth year, and in his academic dissertation (November 28th, 1759), the Theoria generationis, expounded the new theory of a real development on a basis of epigenesis. This treatise is, in spite of its smallness and its obscure phraseology, one of the most valuable in the whole range of biological literature. It is equally distinguished for the mass of new and careful observations it contains, and the far-reaching and pregnant ideas which the author everywhere extracts from his observations and builds into a luminous and accurate theory of generation. Nevertheless, it met with no success at the time. Although scientific studies were then assiduously cultivated owing to the impulse given by Linné—although botanists and zoologists were no longer counted by dozens, but by hundreds, hardly any notice was taken of Wolff’s theory. Even when he established the truth of epigenesis by the most rigorous observations, and demolished the airy structure of the preformation theory, the “exact” scientist Haller proved one of the most strenuous supporters of the old theory, and rejected Wolff’s correct view with a dictatorial “There is no such thing as evolution.” He even went on to say that religion was menaced by the new theory! It is not surprising that the whole of the physiologists of the second half of the eighteenth century submitted to the ruling of this physiological pontiff, and attacked the theory of epigenesis as a dangerous innovation. It was not until more than fifty years afterwards that Wolff’s work was appreciated. Only when Meckel translated into German in 1812 another valuable work of Wolff’s on The Formation of the Alimentary Canal (written in 1768), and called attention to its great importance, did people begin to think of him once more; yet this obscure writer had evinced a profounder insight into the nature of the living organism than any other scientist of the eighteenth century.
Wolff’s idea led to an appreciable advance over the whole field of biology. There is such a vast number of new and important observations and pregnant thoughts in his writings that we have only gradually learned to appreciate them rightly in the course of the nineteenth century. He opened up the true path for research in many directions. In the first place, his theory of epigenesis gave us our first real insight into the nature of embryonic development. He showed convincingly that the development of every organism consists of a series of new formations, and that there is no trace whatever of the complete form either in the ovum or the spermatozoon. On the contrary, these are quite simple bodies, with a very different purport. The embryo which is developed from them is also quite different, in its internal arrangement and outer configuration, from the complete organism. There is no trace whatever of preformation or in-folding of organs. To-day we can scarcely call epigenesis a theory, because we are convinced it is a fact, and can demonstrate it at any moment with the aid of the microscope.
Wolff furnished the conclusive empirical proof of his theory in his classic dissertation on The Formation of the Alimentary Canal (1768). In its complete state the alimentary canal of the hen is a long and complex tube, with which the lungs, liver, salivary glands, and many other small glands, are connected. Wolff showed that in the early stages of the embryonic chick there is no trace whatever of this complicated tube with all its dependencies, but instead of it only a flat, leaf-shaped body; that, in fact, the whole embryo has at first the appearance of a flat, oval-shaped leaf. When we remember how difficult the exact observation of so fine and delicate a structure as the early leaf-shaped body of the chick must have been with the poor microscopes then in use, we must admire the rare faculty for observation which enabled Wolff to make the most important discoveries in this most difficult part of embryology. By this laborious research he reached the correct opinion that the embryonic body of all the higher animals, such as the birds, is for some time merely a flat, thin, leaf-shaped disk—consisting at first of one layer, but afterwards of several. The lowest of these layers is the alimentary canal, and Wolff followed its development from its commencement to its completion. He showed how this leaf-shaped structure first turns into a groove, then the margins of this groove fold together and form a closed canal, and at length the two external openings of the tube (the mouth and anus) appear.
Moreover, the important fact that the other systems of organs are developed in the same way, from tubes formed out of simple layers, did not escape Wolff. The nerveless system, muscular system, and vascular (blood-vessel) system, with all the organs appertaining thereto, are, like the alimentary system, developed out of simple leaf-shaped structures. Hence, Wolff came to the view by 1768 which Pander developed in the Theory of Germinal Layers fifty years afterwards. His principles are not literally correct; but he comes as near to the truth in them as was possible at that time, and could be expected of him.
Our admiration of this gifted genius increases when we find that he was also the precursor of Goethe in regard to the metamorphosis of plants and of the famous cellular theory. Wolff had, as Huxley showed, a clear presentiment of this cardinal theory, since he recognised small microscopic globules as the elementary parts out of which the germinal layers arose.
Finally, I must invite special attention to the mechanical character of the profound philosophic reflections which Wolff always added to his remarkable observations. He was a great monistic philosopher, in the best meaning of the word. It is unfortunate that his philosophic discoveries were ignored as completely as his observations for more than half a century. We must be all the more careful to emphasise the fact of their clear monistic tendency.
We may distinguish three chief periods in the growth of our science of human embryology. The first has been considered in the preceding chapter; it embraces the whole of the preparatory period of research, and extends from Aristotle to Caspar Friedrich Wolff, or to the year 1759, in which the epoch-making Theoria generationis was published. The second period, with which we have now to deal, lasts about a century—that is to say, until the appearance of Darwin’s Origin of Species, which brought about a change in the very foundations of biology, and, in particular, of embryology. The third period begins with Darwin. When we say that the second period lasted a full century, we must remember that Wolff’s work had remained almost unnoticed during half the time—namely, until the year 1812. During the whole of these fifty-three years not a single book that appeared followed up the path that Wolff had opened, or extended his theory of embryonic development. We merely find his views—perfectly correct views, based on extensive observations of fact—mentioned here and there as erroneous; their opponents, who adhered to the dominant theory of preformation, did not even deign to reply to them. This unjust treatment was chiefly due to the extraordinary authority of Albrecht von Haller; it is one of the most astonishing instances of a great authority, as such, preventing for a long time the recognition of established facts.
The general ignorance of Wolff’s work was so great that at the beginning of the nineteenth century two scientists of Jena, Oken (1806) and Kieser (1810), began independent research into the development of the alimentary canal of the chick, and hit upon the right clue to the embryonic puzzle, without knowing a word about Wolff’s important treatise on the same subject. They were treading in his very footsteps without suspecting it. This can be easily proved from the fact that they did not travel as far as Wolff. It was not until Meckel translated into German Wolff’s book on the alimentary system, and pointed out its great importance, that the eyes of anatomists and physiologists were suddenly opened. At once a number of biologists instituted fresh embryological inquiries, and began to confirm Wolff’s theory of epigenesis.
This resuscitation of embryology and development of the epigenesis-theory was chiefly connected with the university of Würtzburg. One of the professors there at that time was Döllinger, an eminent biologist, and father of the famous Catholic historian who later distinguished himself by his opposition to the new dogma of papal infallibility. Döllinger was both a profound thinker and an accurate observer. He took the keenest interest in embryology, and worked at it a good deal. However, he is not himself responsible for any important result in this field. In 1816 a young medical doctor, whom we may at once designate as Wolff’s chief successor, Karl Ernst von Baer, came to Würtzburg. Baer’s conversations with Döllinger on embryology led to a fresh series of most extensive investigations. Döllinger had expressed a wish that some young scientist should begin again under his guidance an independent inquiry into the development of the chick during the hatching of the egg. As neither he nor Baer had money enough to pay for an incubator and the proper control of the experiments, and for a competent artist to illustrate the various stages observed, the lead of the enterprise was given to Christian Pander, a wealthy friend of Baer’s who had been induced by Baer to come to Würtzburg. An able engraver, Dalton, was engaged to do the copper-plates. In a short time the embryology of the chick, in which Baer was taking the greatest indirect interest, was so far advanced that Pander was able to sketch the main features of it on the ground of Wolff’s theory in the dissertation he published in 1817. He clearly enunciated the theory of germinal layers which Wolff had anticipated, and established the truth of Wolff’s idea of a development of the complicated systems of organs out of simple leaf-shaped primitive structures. According to Pander, the leaf-shaped object in the hen’s egg divides, before the incubation has proceeded twelve hours, into two different layers, an external serous layer and an internal mucous layer; between the two there develops later a third layer, the vascular (blood-vessel) layer.[8]
[8] The technical terms which are bound to creep into this chapter will be fully understood later on.—Translator.
Karl Ernst von Baer, who had set afoot Pander’s investigation, and had shown the liveliest interest in it after Pander’s departure from Würtzburg, began his own much more comprehensive research in 1819. He published the mature result nine years afterwards in his famous work, Animal Embryology: Observation and Reflection (not translated). This classic work still remains a model of careful observation united to profound philosophic speculation. The first part appeared in 1828, the second in 1837. The book proved to be the foundation on which the whole science of embryology has built down to our own day. It so far surpassed its predecessors, and Pander in particular, that it has become, after Wolff’s work, the chief base of modern embryology.
Baer was one of the greatest scientists of the nineteenth century, and exercised considerable influence on other branches of biology as well. He built up the theory of germinal layers, as a whole and in detail, so clearly and solidly that it has been the starting-point of embryological research ever since. He taught that in all the vertebrates first two and then four of these germinal layers are formed; and that the earliest rudimentary organs of the body arise by the conversion of these layers into tubes. He described the first appearance of the vertebrate embryo, as it may be seen in the globular yelk of the fertilised egg, as an oval disk which first divides into two layers. From the upper or animal layer are developed all the organs which accomplish the phenomena of animal life—the functions of sensation and motion, and the covering of the body. From the lower or vegetative layer come the organs which effect the vegetative life of the organism—nutrition, digestion, blood-formation, respiration, secretion, reproduction, etc.
Each of these original layers divides, according to Baer, into two thinner and superimposed layers or plates. He calls the two plates of the animal layer, the skin-stratum and muscle-stratum. From the upper of these plates, the skin-stratum, the external skin, or outer covering of the body, the central nervous system, and the sense-organs, are formed. From the lower, or muscle-stratum, the muscles, or fleshy parts and the bony skeleton—in a word, the motor organs—are evolved. In the same way, Baer said, the lower or vegetative layer splits into two plates, which he calls the vascular-stratum and the mucous-stratum. From the outer of the two (the vascular) the heart, blood-vessels, spleen, and the other vascular glands, the kidneys, and sexual glands, are formed. From the fourth or mucous layer, in fine, we get the internal and digestive lining of the alimentary canal and all its dependencies, the liver, lungs, salivary glands, etc. Baer had, in the main, correctly judged the significance of these four secondary embryonic layers, and he followed the conversion of them into the tube-shaped primitive organs with great perspicacity. He first solved the difficult problem of the transformation of this four-fold, flat, leaf-shaped, embryonic disk into the complete vertebrate body, through the conversion of the layers or plates into tubes. The flat leaves bend themselves in obedience to certain laws of growth; the borders of the curling plates approach nearer and nearer; until at last they come into actual contact. Thus out of the flat gut-plate is formed a hollow gut-tube, out of the flat spinal plate a hollow nerve-tube, from the skin-plate a skin-tube, and so on.
Among the many great services which Baer rendered to embryology, especially vertebrate embryology, we must not forget his discovery of the human ovum. Earlier scientists had, as a rule, of course, assumed that man developed out of an egg, like the other animals. In fact, the preformation theory held that the germs of the whole of humanity were stored already in Eve’s ova. But the real ovum escaped detection until the year 1827. This ovum is extremely small, being a tiny round vesicle about the 1/120 of an inch in diameter; it can be seen under very favourable circumstances with the naked eye as a tiny particle, but is otherwise quite invisible. This particle is formed in the ovary inside a much larger globule, which takes the name of the Graafian follicle, from its discoverer, Graaf, and had previously been regarded as the true ovum. However, in 1827 Baer proved that it was not the real ovum, which is much smaller, and is contained within the follicle. (Compare the end of Chapter XXIX.)
Baer was also the first to observe what is known as the segmentation sphere of the vertebrate; that is to say, the round vesicle which first develops out of the impregnated ovum, and the thin wall of which is made up of a single layer of regular, polygonal (many-cornered) cells (see the illustration in Chapter XII). Another discovery of his that was of great importance in constructing the vertebrate stem and the characteristic organisation of this extensive group (to which man belongs) was the detection of the axial rod, or the chorda dorsalis. There is a long, round, cylindrical rod of cartilage which runs down the longer axis of the vertebrate embryo; it appears at an early stage, and is the first sketch of the spinal column, the solid skeletal axis of the vertebrate. In the lowest of the vertebrates, the amphioxus, the internal skeleton consists only of this cord throughout life. But even in the case of man and all the higher vertebrates it is round this cord that the spinal column and the brain are afterwards formed.
However, important as these and many other discoveries of Baer’s were in vertebrate embryology, his researches were even more influential, from the circumstance that he was the first to employ the comparative method in studying the development of the animal frame. Baer occupied himself chiefly with the embryology of vertebrates (especially the birds and fishes). But he by no means confined his attention to these, gradually taking the various groups of the invertebrates into his sphere of study. As the general result of his comparative embryological research, Baer distinguished four different modes of development and four corresponding groups in the animal world. These chief groups or types are: 1, the vertebrata; 2, the articulata; 3, the mollusca; and 4, all the lower groups which were then wrongly comprehended under the general name of the radiata. Georges Cuvier had been the first to formulate this distinction, in 1812. He showed that these groups present specific differences in their whole internal structure, and the connection and disposal of their systems of organs; and that, on the other hand, all the animals of the same type—say, the vertebrates—essentially agreed in their inner structure, in spite of the greatest superficial differences. But Baer proved that these four groups are also quite differently developed from the ovum; and that the series of embryonic forms is the same throughout for animals of the same type, but different in the case of other animals. Up to that time the chief aim in the classification of the animal kingdom was to arrange all the animals from lowest to highest, from the infusorium to man, in one long and continuous series. The erroneous idea prevailed nearly everywhere that there was one uninterrupted chain of evolution from the lowest animal to the highest. Cuvier and Baer proved that this view was false, and that we must distinguish four totally different types of animals, on the ground of anatomic structure and embryonic development.
Baer’s epoch-making works aroused an extraordinary and widespread interest in embryological research. Immediately afterwards we find a great number of observers at work in the newly opened field, enlarging it in a very short time with great energy by their various discoveries in detail. Next to Baer’s comes the admirable work of Heinrich Rathke, of Königsberg (died 1860); he made an extensive study of the embryology, not only of the invertebrates (crustaceans, insects, molluscs), but also, and particularly, of the vertebrates (fishes, tortoises, serpents, crocodiles, etc.). We owe the first comprehensive studies of mammal embryology to the careful research of Wilhelm Bischoff, of Munich; his embryology of the rabbit (1840), the dog (1842), the guinea-pig (1852), and the doe (1854), still form classical studies. About the same time a great impetus was given to the embryology of the invertebrates. The way was opened through this obscure province by the studies of the famous Berlin zoologist, Johannes Müller, on the echinoderms. He was followed by Albert Kölliker, of Würtzburg, writing on the cuttlefish (or the cephalopods), Siebold and Huxley on worms and zoophytes, Fritz Muller (Desterro) on the crustacea, Weismann on insects, and so on. The number of workers in this field has greatly increased of late, and a quantity of new and astonishing discoveries have been made. One notices, in several of these recent works on embryology, that their authors are too little acquainted with comparative anatomy and classification. Paleontology is, unfortunately, altogether neglected by many of these new workers, although this interesting science furnishes most important facts for phylogeny, and thus often proves of very great service in ontogeny.
A very important advance was made in our science in 1839, when the cellular theory was established, and a new field of inquiry bearing on embryology was suddenly opened. When the famous botanist, M. Schleiden, of Jena, showed in 1838, with the aid of the microscope, that every plant was made up of innumerable elementary parts, which we call cells, a pupil of Johannes Müller at Berlin, Theodor Schwann, applied the discovery at once to the animal organism. He showed that in the animal body as well, when we examine its tissues in the microscope, we find these cells everywhere to be the elementary units. All the different tissues of the organism, especially the very dissimilar tissues of the nerves, muscles, bones, external skin, mucous lining, etc., are originally formed out of cells; and this is also true of all the tissues of the plant. These cells are separate living beings; they are the citizens of the State which the entire multicellular organism seems to be. This important discovery was bound to be of service to embryology, as it raised a number of new questions. What is the relation of the cells to the germinal layers? Are the germinal layers composed of cells, and what is their relation to the cells of the tissues that form later? How does the ovum stand in the cellular theory? Is the ovum itself a cell, or is it composed of cells? These important questions were now imposed on the embryologist by the cellular theory.
The most notable effort to answer these questions—which were attacked on all sides by different students—is contained in the famous work, Inquiries into the Development of the Vertebrates (not translated) of Robert Remak, of Berlin (1851). This gifted scientist succeeded in mastering, by a complete reform of the science, the great difficulties which the cellular theory had at first put in the way of embryology. A Berlin anatomist, Carl Boguslaus Reichert, had already attempted to explain the origin of the tissues. But this attempt was bound to miscarry, since its not very clear-headed author lacked a sound acquaintance with embryology and the cell theory, and even with the structure and development of the tissue in particular. Remak at length brought order into the dreadful confusion that Reichert had caused; he gave a perfectly simple explanation of the origin of the tissues. In his opinion the animal ovum is always a simple cell : the germinal layers which develop out of it are always composed of cells; and these cells that constitute the germinal layers arise simply from the continuous and repeated cleaving (segmentation) of the original solitary cell. It first divides into two and then into four cells; out of these four cells are born eight, then sixteen, thirty-two, and so on. Thus, in the embryonic development of every animal and plant there is formed first of all out of the simple egg cell, by a repeated subdivision, a cluster of cells, as Kölliker had already stated in connection with the cephalopods in 1844. The cells of this group spread themselves out flat and form leaves or plates; each of these leaves is formed exclusively out of cells. The cells of different layers assume different shapes, increase, and differentiate; and in the end there is a further cleavage (differentiation) and division of work of the cells within the layers, and from these all the different tissues of the body proceed.
These are the simple foundations of histogeny, or the science that treats of the development of the tissues ( hista), as it was established by Remak and Kölliker. Remak, in determining more closely the part which the different germinal layers play in the formation of the various tissues and organs, and in applying the theory of evolution to the cells and the tissues they compose, raised the theory of germinal layers, at least as far as it regards the vertebrates, to a high degree of perfection.
Remak showed that three layers are formed out of the two germinal layers which compose the first simple leaf-shaped structure of the vertebrate body (or the “germinal disk”), as the lower layer splits into two plates. These three layers have a very definite relation to the various tissues. First of all, the cells which form the outer skin of the body (the epidermis), with its various dependencies (hairs, nails, etc.)—that is to say, the entire outer envelope of the body—are developed out of the outer or upper layer; but there are also developed in a curious way out of the same layer the cells which form the central nervous system, the brain and the spinal cord. In the second place, the inner or lower germinal layer gives rise only to the cells which form the epithelium (the whole inner lining) of the alimentary canal and all that depends on it (the lungs, liver, pancreas, etc.), or the tissues that receive and prepare the nourishment of the body. Finally, the middle layer gives rise to all the other tissues of the body, the muscles, blood, bones, cartilage, etc. Remak further proved that this middle layer, which he calls “the motor-germinative layer,” proceeds to subdivide into two secondary layers. Thus we find once more the four layers which Baer had indicated. Remak calls the outer secondary leaf of the middle layer (Baer’s “muscular layer”) the “skin layer” (it would be better to say, skin-fibre layer); it forms the outer wall of the body (the true skin, the muscles, etc.). To the inner secondary leaf (Baer’s “vascular layer”) he gave the name of the “alimentary-fibre layer”; this forms the outer envelope of the alimentary canal, with the mesentery, the heart, the blood-vessels, etc.
On this firm foundation provided by Remak for histogeny, or the science of the formation of the tissues, our knowledge has been gradually built up and enlarged in detail. There have been several attempts to restrict and even destroy Remak’s principles. The two anatomists, Reichert (of Berlin) and Wilhelm His (of Leipzic), especially, have endeavoured in their works to introduce a new conception of the embryonic development of the vertebrate, according to which the two primary germinal layers would not be the sole sources of formation. But these efforts were so seriously marred by ignorance of comparative anatomy, an imperfect acquaintance with ontogenesis, and a complete neglect of phylogenesis, that they could not have more than a passing success. We can only explain how these curious attacks of Reichert and His came to be regarded for a time as advances by the general lack of discrimination and of grasp of the true object of embryology.
Wilhelm His published, in 1868, his extensive Researches into the Earliest Form of the Vertebrate Body,[9] one of the curiosities of embryological literature. The author imagines that he can build a “mechanical theory of embryonic development” by merely giving an exact description of the embryology of the chick, without any regard to comparative anatomy and phylogeny, and thus falls into an error that is almost without parallel in the history of biological literature. As the final result of his laborious investigations, His tells us “that a comparatively simple law of growth is the one essential thing in the first development. Every formation, whether it consist in cleavage of layers, or folding, or complete division, is a consequence of this fundamental law.” Unfortunately, he does not explain what this “law of growth” is; just as other opponents of the theory of selection, who would put in its place a great “law of evolution,” omit to tell us anything about the nature of this. Nevertheless, it is quite clear from His’s works that he imagines constructive Nature to be a sort of skilful tailor. The ingenious operator succeeds in bringing into existence, by “evolution,” all the various forms of living things by cutting up in different ways the germinal layers, bending and folding, tugging and splitting, and so on.
[9] None of His’s works have been translated into English.
His’s embryological theories excited a good deal of interest at the time of publication, and have evoked a fair amount of literature in the last few decades. He professed to explain the most complicated parts of organic construction (such as the development of the brain) in the simplest way on mechanical principles, and to derive them immediately from simple physical processes (such as unequal distribution of strain in an elastic plate). It is quite true that a mechanical or monistic explanation (or a reduction of natural processes) is the ideal of modern science, and this ideal would be realised if we could succeed in expressing these formative processes in mathematical formulæ. His has, therefore, inserted plenty of numbers and measurements in his embryological works, and given them an air of “exact” scholarship by putting in a quantity of mathematical tables. Unfortunately, they are of no value, and do not help us in the least in forming an “exact” acquaintance with the embryonic phenomena. Indeed, they wander from the true path altogether by neglecting the phylogenetic method; this, he thinks, is “a mere by-path,” and is “not necessary at all for the explanation of the facts of embryology,” which are the direct consequence of physiological principles. What His takes to be a simple physical process—for instance, the folding of the germinal layers (in the formation of the medullary tube, alimentary tube, etc.)—is, as a matter of fact, the direct result of the growth of the various cells which form those organic structures; but these growth-motions have themselves been transmitted by heredity from parents and ancestors, and are only the hereditary repetition of countless phylogenetic changes which have taken place for thousands of years in the race-history of the said ancestors. Each of these historical changes was, of course, originally due to adaptation; it was, in other words, physiological, and reducible to mechanical causes. But we have, naturally, no means of observing them now. It is only by the hypotheses of the science of evolution that we can form an approximate idea of the organic links in this historic chain.
All the best recent research in animal embryology has led to the confirmation and development of Baer and Remak’s theory of the germinal layers. One of the most important advances in this direction of late was the discovery that the two primary layers out of which is built the body of all vertebrates (including man) are also present in all the invertebrates, with the sole exception of the lowest group, the unicellular protozoa. Huxley had detected them in the medusa in 1849. He showed that the two layers of cells from which the body of this zoophyte is developed correspond, both morphologically and physiologically, to the two original germinal layers of the vertebrate. The outer layer, from which come the external skin and the muscles, was then called by Allman (1853) the “ectoderm” (outer layer, or skin); the inner layer, which forms the alimentary and reproductory organs, was called the “entoderm” (= inner layer). In 1867 and the following years the discovery of the germinal layers was extended to other groups of the invertebrates. In particular, the indefatigable Russian zoologist, Kowalevsky, found them in all the most diverse sections of the invertebrates—the worms, tunicates, echinoderms, molluscs, articulates, etc.
In my monograph on the sponges (1872) I proved that these two primary germinal layers are also found in that group, and that they may be traced from it right up to man, through all the various classes, in identical form. This “homology of the two primary germinal layers” extends through the whole of the metazoa, or tissue-forming animals; that is to say, through the whole animal kingdom, with the one exception of its lowest section, the unicellular beings, or protozoa. These lowly organised animals do not form germinal layers, and therefore do not succeed in forming true tissue. Their whole body consists of a single cell (as is the case with the amœbæ and infusoria), or of a loose aggregation of only slightly differentiated cells, though it may not even reach the full structure of a single cell (as with the monera). But in all other animals the ovum first grows into two primary layers, the outer or animal layer (the ectoderm, epiblast, or ectoblast), and the inner or vegetal layer (the entoderm, hypoblast, or endoblast); and from these the tissues and organs are formed. The first and oldest organ of all these metazoa is the primitive gut (or progaster) and its opening, the primitive mouth (prostoma). The typical embryonic form of the metazoa, as it is presented for a time by this simple structure of the two-layered body, is called the gastrula ; it is to be conceived as the hereditary reproduction of some primitive common ancestor of the metazoa, which we call the gastræa. This applies to the sponges and other zoophyta, and to the worms, the mollusca, echinoderma, articulata, and vertebrata. All these animals may be comprised under the general heading of “gut animals,” or metazoa, in contradistinction to the gutless protozoa.
I have pointed out in my Study of the Gastræa Theory [not translated] (1873) the important consequences of this conception in the morphology and classification of the animal world. I also divided the realm of metazoa into two great groups, the lower and higher metazoa. In the first are comprised the cœlenterata (also called zoophytes, or plant-animals). In the lower forms of this group the body consists throughout life merely of the primary germinal layers, with the cells sometimes more and sometimes less differentiated. But with the higher forms of the cœlentarata (the corals, higher medusæ, ctenophoræ, and platodes) a middle layer, or mesoderm, often of considerable size, is developed between the other two layers; but blood and an internal cavity are still lacking.
To the second great group of the metazoa I gave the name of the cœlomaria, or bilaterata (or the bilateral higher forms). They all have a cavity within the body (cœloma), and most of them have blood and blood-vessels. In this are comprised the six higher stems of the animal kingdom, the annulata and their descendants, the mollusca, echinoderma, articulata, tunicata, and vertebrata. In all these bilateral organisms the two-sided body is formed out of four secondary germinal layers, of which the inner two construct the wall of the alimentary canal, and the outer two the wall of the body. Between the two pairs of layers lies the cavity (cœloma).
Although I laid special stress on the great morphological importance of this cavity in my Study of the Gastræa Theory, and endeavoured to prove the significance of the four secondary germinal layers in the organisation of the cœlomaria, I was unable to deal satisfactorily with the difficult question of the mode of their origin. This was done eight years afterwards by the brothers Oscar and Richard Hertwig in their careful and extensive comparative studies. In their masterly Cœlum Theory: An Attempt to Explain the Middle Germinal Layer [not translated] (1881) they showed that in most of the metazoa, especially in all the vertebrates, the body-cavity arises in the same way, by the outgrowth of two sacs from the inner layer. These two cœlom-pouches proceed from the rudimentary mouth of the gastrula, between the two primary layers. The inner plate of the two-layered cœlom-pouch (the visceral layer) joins itself to the entoderm; the outer plate (parietal layer) unites with the ectoderm. Thus are formed the double-layered gut-wall within and the double-layered body-wall without; and between the two is formed the cavity of the cœlom, by the blending of the right and left cœlom-sacs. We shall see this more fully in Chapter X.
The many new points of view and fresh ideas suggested by my gastræa theory and Hertwig’s cœlom theory led to the publication of a number of writings on the theory of germinal layers. Most of them set out to oppose it at first, but in the end the majority supported it. Of late years both theories are accepted in their essential features by nearly every competent man of science, and light and order have been introduced into this once dark and contradictory field of research. A further cause of congratulation for this solution of the great embryological controversy is that it brought with it a recognition of the need for phylogenetic study and explanation.
Interest and practice in embryological research have been remarkably stimulated during the past thirty years by this appreciation of phylogenetic methods. Hundreds of assiduous and able observers are now engaged in the development of comparative embryology and its establishment on a basis of evolution, whereas they numbered only a few dozen not many decades ago. It would take too long to enumerate even the most important of the countless valuable works which have enriched embryological literature since that time. References to them will be found in the latest manuals of embryology of Kölliker, Balfour, Hertwig, Kollman, Korschelt, and Heider.
Kölliker’s Entwickelungsgeschichte des Menschen und der höherer Thiere, the first edition of which appeared forty-two years ago, had the rare merit at that time of gathering into presentable form the scattered attainments of the science, and expounding them in some sort of unity on the basis of the cellular theory and the theory of germinal layers. Unfortunately, the distinguished Würtzburg anatomist, to whom comparative anatomy, histology, and ontogeny owe so much, is opposed to the theory of descent generally and to Darwinism in particular. All the other manuals I have mentioned take a decided stand on evolution. Francis Balfour has carefully collected and presented with discrimination, in his Manual of Comparative Embryology (1880), the very scattered and extensive literature of the subject; he has also widened the basis of the gastræa theory by a comparative description of the rise of the organs from the germinal layers in all the chief groups of the animal kingdom, and has given a most thorough empirical support to the principles I have formulated. A comparison of his work with the excellent Text-book of the Embryology of the Vertebrates (1890) [translation 1895] of Korschelt and Heider shows what astonishing progress has been made in the science in the course of ten years. I would especially recommend the manuals of Julius Kollmann and Oscar Hertwig to those readers who are stimulated to further study by these chapters on human embryology. Kollmann’s work is commendable for its clear treatment of the subject and very fine original illustrations; its author adheres firmly to the biogenetic law, and uses it throughout with considerable profit. That is not the case in Oscar Hertwig’s recent Text-book of the Embryology of Man and the Mammals [translations 1892 and 1899] (seventh edition 1902). This able anatomist has of late often been quoted as an opponent of the biogenetic law, although he himself had demonstrated its great value thirty years ago. His recent vacillation is partly due to the timidity which our “exact” scientists have with regard to hypotheses; though it is impossible to make any headway in the explanation of facts without them. However, the purely descriptive part of embryology in Hertwig’s Text-book is very thorough and reliable.
A new branch of embryological research has been studied very assiduously in the last decade of the nineteenth century—namely, “experimental embryology.” The great importance which has been attached to the application of physical experiments to the living organism for the last hundred years, and the valuable results that it has given to physiology in the study of the vital phenomena, have led to its extension to embryology. I was the first to make experiments of this kind during a stay of four months on the Canary Island, Lanzerote, in 1866. I there made a thorough investigation of the almost unknown embryology of the siphonophoræ. I cut a number of the embryos of these animals (which develop freely in the water, and pass through a very curious transformation), at an early stage, into several pieces, and found that a fresh organism (more or less complete, according to the size of the piece) was developed from each particle. More recently some of my pupils have made similar experiments with the embryos of vertebrates (especially the frog) and some of the invertebrates. Wilhelm Roux, in particular, has made extensive experiments, and based on them a special “mechanical embryology,” which has given rise to a good deal of discussion and controversy. Roux has published a special journal for these subjects since 1895, the Archiv für Entwickelungsmechanik. The contributions to it are very varied in value. Many of them are valuable papers on the physiology and pathology of the embryo. Pathological experiments—the placing of the embryo in abnormal conditions—have yielded many interesting results; just as the physiology of the normal body has for a long time derived assistance from the pathology of the diseased organism. Other of these mechanical-embryological articles return to the erroneous methods of His, and are only misleading. This must be said of the many contributions of mechanical embryology which take up a position of hostility to the theory of descent and its chief embryological foundation—the biogenetic law. This law, however, when rightly understood, is not opposed to, but is the best and most solid support of, a sound mechanical embryology. Impartial reflection and a due attention to paleontology and comparative anatomy should convince these one-sided mechanicists that the facts they have discovered—and, indeed, the whole embryological process—cannot be fully understood without the theory of descent and the biogenetic law.
The embryology of man and the animals, the history of which we have reviewed in the last two chapters, was mainly a descriptive science forty years ago. The earlier investigations in this province were chiefly directed to the discovery, by careful observation, of the wonderful facts of the embryonic development of the animal body from the ovum. Forty years ago no one dared attack the question of the causes of these phenomena. For fully a century, from the year 1759, when Wolff’s solid Theoria generationis appeared, until 1859, when Darwin published his famous Origin of Species, the real causes of the embryonic processes were quite unknown. No one thought of seeking the agencies that effected this marvellous succession of structures. The task was thought to be so difficult as almost to pass beyond the limits of human thought. It was reserved for Charles Darwin to initiate us into the knowledge of these causes. This compels us to recognise in this great genius, who wrought a complete revolution in the whole field of biology, a founder at the same time of a new period in embryology. It is true that Darwin occupied himself very little with direct embryological research, and even in his chief work he only touches incidentally on the embryonic phenomena; but by his reform of the theory of descent and the founding of the theory of selection he has given us the means of attaining to a real knowledge of the causes of embryonic formation. That is, in my opinion, the chief feature in Darwin’s incalculable influence on the whole science of evolution.
When we turn our attention to this latest period of embryological research, we pass into the second division of organic evolution—stem-evolution, or phylogeny. I have already indicated in Chapter I the important and intimate causal connection between these two sections of the science of evolution—between the evolution of the individual and that of his ancestors. We have formulated this connection in the biogenetic law; the shorter evolution, that of the individual, or ontogenesis, is a rapid and summary repetition, a condensed recapitulation, of the larger evolution, or that of the species. In this principle we express all the essential points relating to the causes of evolution; and we shall seek throughout this work to confirm this principle and lend it the support of facts. When we look to its causal significance, perhaps it would be better to formulate the biogenetic law thus: “The evolution of the species and the stem ( phylon) shows us, in the physiological functions of heredity and adaptation, the conditioning causes on which the evolution of the individual depends”; or, more briefly: “Phylogenesis is the mechanical cause of ontogenesis.”
But before we examine the great achievement by which Darwin revealed the causes of evolution to us, we must glance at the efforts of earlier scientists to attain this object. Our historical inquiry into these will be even shorter than that into the work done in the field of ontogeny. We have very few names to consider here. At the head of them we find the great French naturalist, Jean Lamarck, who first established evolution as a scientific theory in 1809. Even before his time, however, the chief philosopher, Kant, and the chief poet, Goethe, of Germany had occupied themselves with the subject. But their efforts passed almost without recognition in the eighteenth century. A “philosophy of nature” did not arise until the beginning of the nineteenth century. In the whole of the time before this no one had ventured to raise seriously the question of the origin of species, which is the culminating point of phylogeny. On all sides it was regarded as an insoluble enigma.
The whole science of the evolution of man and the other animals is intimately connected with the question of the nature of species, or with the problem of the origin of the various animals which we group together under the name of species. Thus the definition of the species becomes important. It is well known that this definition was given by Linné, who, in his famous Systema Naturæ (1735), was the first to classify and name the various groups of animals and plants, and drew up an orderly scheme of the species then known. Since that time “species” has been the most important and indispensable idea in descriptive natural history, in zoological and botanical classification; although there have been endless controversies as to its real meaning.
What, then, is this “organic species”? Linné himself appealed directly to the Mosaic narrative; he believed that, as it is stated in Genesis, one pair of each species of animals and plants was created in the beginning, and that all the individuals of each species are the descendants of these created couples. As for the hermaphrodites (organisms that have male and female organs in one being), he thought it sufficed to assume the creation of one sole individual, since this would be fully competent to propagate its species. Further developing these mystic ideas, Linné went on to borrow from Genesis the account of the deluge and of Noah’s ark as a ground for a science of the geographical and topographical distribution of organisms. He accepted the story that all the plants, animals, and men on the earth were swept away in a universal deluge, except the couples preserved with Noah in the ark, and ultimately landed on Mount Ararat. This mountain seemed to Linné particularly suitable for the landing, as it reaches a height of more than 16,000 feet, and thus provides in its higher zones the several climates demanded by the various species of animals and plants: the animals that were accustomed to a cold climate could remain at the summit; those used to a warm climate could descend to the foot; and those requiring a temperate climate could remain half-way down. From this point the re-population of the earth with animals and plants could proceed.
It was impossible to have any scientific notion of the method of evolution in Linné’s time, as one of the chief sources of information, paleontology, was still wholly unknown. This science of the fossil remains of extinct animals and plants is very closely bound up with the whole question of evolution. It is impossible to explain the origin of living organisms without appealing to it. But this science did not rise until a much later date. The real founder of scientific paleontology was Georges Cuvier, the most distinguished zoologist who, after Linné, worked at the classification of the animal world, and effected a complete revolution in systematic zoology at the beginning of the nineteenth century. In regard to the nature of the species he associated himself with Linné and the Mosaic story of creation, though this was more difficult for him with his acquaintance with fossil remains. He clearly showed that a number of quite different animal populations have lived on the earth; and he claimed that we must distinguish a number of stages in the history of our planet, each of which was characterised by a special population of animals and plants. These successive populations were, he said, quite independent of each other, and therefore the supernatural creative act, which was demanded as the origin of the animals and plants by the dominant creed, must have been repeated several times. In this way a whole series of different creative periods must have succeeded each other; and in connection with these he had to assume that stupendous revolutions or cataclysms—something like the legendary deluge—must have taken place repeatedly. Cuvier was all the more interested in these catastrophes or cataclysms as geology was just beginning to assert itself, and great progress was being made in our knowledge of the structure and formation of the earth’s crust. The various strata of the crust were being carefully examined, especially by the famous geologist Werner and his school, and the fossils found in them were being classified; and these researches also seemed to point to a variety of creative periods. In each period the earth’s crust, composed of the various strata, seemed to be differently constituted, just like the population of animals and plants that then lived on it. Cuvier combined this notion with the results of his own paleontological and zoological research; and in his effort to get a consistent view of the whole process of the earth’s history he came to form the theory which is known as “the catastrophic theory,” or the theory of terrestrial revolutions. According to this theory, there have been a series of mighty cataclysms on the earth, and these have suddenly destroyed the whole animal and plant population then living on it; after each cataclysm there was a fresh creation of living things throughout the earth. As this creation could not be explained by natural laws, it was necessary to appeal to an intervention on the part of the Creator. This catastrophic theory, which Cuvier described in a special work, was soon generally accepted, and retained its position in biology for half a century.
However, Cuvier’s theory was completely overthrown sixty years ago by the geologists, led by Charles Lyell, the most distinguished worker in this field of science. Lyell proved in his famous Principles of Geology (1830) that the theory was false, in so far as it concerned the crust of the earth; that it was totally unnecessary to bring in supernatural agencies or general catastrophes in order to explain the structure and formation of the mountains; and that we can explain them by the familiar agencies which are at work to-day in altering and reconstructing the surface of the earth. These causes are—the action of the atmosphere and water in its various forms (snow, ice, fog, rain, the wear of the river, and the stormy ocean), and the volcanic action which is exerted by the molten central mass. Lyell convincingly proved that these natural causes are quite adequate to explain every feature in the build and formation of the crust. Hence Cuvier’s theory of cataclysms was very soon driven out of the province of geology, though it remained for another thirty years in undisputed authority in biology. All the zoologists and botanists who gave any thought to the question of the origin of organisms adhered to Cuvier’s erroneous idea of revolutions and new creations.
In order to illustrate the complete stagnancy of biology from 1830 to 1859 on the question of the origin of the various species of animals and plants, I may say, from my own experience, that during the whole of my university studies I never heard a single word said about this most important problem of the science. I was fortunate enough at that time (1852–1857) to have the most distinguished masters for every branch of biological science. Not one of them ever mentioned this question of the origin of species. Not a word was ever said about the earlier efforts to understand the formation of living things, nor about Lamarck’s Philosophie Zoologique which had made a fresh attack on the problem in 1809. Hence it is easy to understand the enormous opposition that Darwin encountered when he took up the question for the first time. His views seemed to float in the air, without a single previous effort to support them. The whole question of the formation of living things was considered by biologists, until 1859, as pertaining to the province of religion and transcendentalism; even in speculative philosophy, in which the question had been approached from various sides, no one had ventured to give it serious treatment. This was due to the dualistic system of Immanuel Kant, who taught a natural system of evolution as far as the inorganic world was concerned; but, on the whole, adopted a supernaturalist system as regards the origin of living things. He even went so far as to say: “It is quite certain that we cannot even satisfactorily understand, much less explain, the nature of an organism and its internal forces on purely mechanical principles; it is so certain, indeed, that we may confidently say: ‘It is absurd for a man to imagine even that some day a Newton will arise who will explain the origin of a single blade of grass by natural laws not controlled by design’—such a hope is entirely forbidden us.” In these words Kant definitely adopts the dualistic and teleological point of view for biological science.
Nevertheless, Kant deserted this point of view at times, particularly in several remarkable passages which I have dealt with at length in my Natural History of Creation (chap. v), where he expresses himself in the opposite, or monistic, sense. In fact, these passages would justify one, as I showed, in claiming his support for the theory of evolution. However, these monistic passages are only stray gleams of light; as a rule, Kant adheres in biology to the obscure dualistic ideas, according to which the forces at work in inorganic nature are quite different from those of the organic world. This dualistic system prevails in academic philosophy to-day—most of our philosophers still regarding these two provinces as totally distinct. They put, on the one side, the inorganic or “lifeless” world, in which there are at work only mechanical laws, acting necessarily and without design; and, on the other, the province of organic nature, in which none of the phenomena can be properly understood, either as regards their inner nature or their origin, except in the light of preconceived design, carried out by final or purposive causes.
The prevalence of this unfortunate dualistic prejudice prevented the problem of the origin of species, and the connected question of the origin of man, from being regarded by the bulk of people as a scientific question at all until 1859. Nevertheless, a few distinguished students, free from the current prejudice, began, at the commencement of the nineteenth century, to make a serious attack on the problem. The merit of this attaches particularly to what is known as “the older school of natural philosophy,” which has been so much misrepresented, and which included Jean Lamarck, Buffon, Geoffroy St. Hilaire, and Blainville in France; Wolfgang Goethe, Reinhold Treviranus, Schelling, and Lorentz Oken in Germany [and Erasmus Darwin in England].
The gifted natural philosopher who treated this difficult question with the greatest sagacity and comprehensiveness was Jean Lamarck. He was born at Bazentin, in Picardy, on August 1st, 1744; he was the son of a clergyman, and was destined for the Church. But he turned to seek glory in the army, and eventually devoted himself to science.
His Philosophie Zoologique was the first scientific attempt to sketch the real course of the origin of species, the first “natural history of creation” of plants, animals, and men. But, as in the case of Wolff’s book, this remarkably able work had no influence whatever; neither one nor the other could obtain any recognition from their prejudiced contemporaries. No man of science was stimulated to take an interest in the work, and to develop the germs it contained of the most important biological truths. The most distinguished botanists and zoologists entirely rejected it, and did not even deign to reply to it. Cuvier, who lived and worked in the same city, has not thought fit to devote a single syllable to this great achievement in his memoir on progress in the sciences, in which the pettiest observations found a place. In short, Lamarck’s Philosophie Zoologique shared the fate of Wolff’s theory of development, and was for half a century ignored and neglected. The German scientists, especially Oken and Goethe, who were occupied with similar speculations at the same time, seem to have known nothing about Lamarck’s work. If they had known it, they would have been greatly helped by it, and might have carried the theory of evolution much farther than they found it possible to do.
To give an idea of the great importance of the Philosophie Zoologique, I will briefly explain Lamarck’s leading thought. He held that there was no essential difference between living and lifeless beings. Nature is one united and connected system of phenomena; and the forces which fashion the lifeless bodies are the only ones at work in the kingdom of living things. We have, therefore, to use the same method of investigation and explanation in both provinces. Life is only a physical phenomenon. All the plants and animals, with man at their head, are to be explained, in structure and life, by mechanical or efficient causes, without any appeal to final causes, just as in the case of minerals and other inorganic bodies. This applies equally to the origin of the various species. We must not assume any original creation, or repeated creations (as in Cuvier’s theory), to explain this, but a natural, continuous, and necessary evolution. The whole evolutionary process has been uninterrupted. All the different kinds of animals and plants which we see to-day, or that have ever lived, have descended in a natural way from earlier and different species; all come from one common stock, or from a few common ancestors. These remote ancestors must have been quite simple organisms of the lowest type, arising by spontaneous generation from inorganic matter. The succeeding species have been constantly modified by adaptation to their varying environment (especially by use and habit), and have transmitted their modifications to their successors by heredity.
Lamarck was the first to formulate as a scientific theory the natural origin of living things, including man, and to push the theory to its extreme conclusions—the rise of the earliest organisms by spontaneous generation (or abiogenesis) and the descent of man from the nearest related mammal, the ape. He sought to explain this last point, which is of especial interest to us here, by the same agencies which he found at work in the natural origin of the plant and animal species. He considered use and habit (adaptation) on the one hand, and heredity on the other, to be the chief of these agencies. The most important modifications of the organs of plants and animals are due, in his opinion, to the function of these very organs, or to the use or disuse of them. To give a few examples, the woodpecker and the humming-bird have got their peculiarly long tongues from the habit of extracting their food with their tongues from deep and narrow folds or canals; the frog has developed the web between his toes by his own swimming; the giraffe has lengthened his neck by stretching up to the higher branches of trees, and so on. It is quite certain that this use or disuse of organs is a most important factor in organic development, but it is not sufficient to explain the origin of species.
To adaptation we must add heredity as the second and not less important agency, as Lamarck perfectly recognised. He said that the modification of the organs in any one individual by use or disuse was slight, but that it was increased by accumulation in passing by heredity from generation to generation. But he missed altogether the principle which Darwin afterwards found to be the chief factor in the theory of transformation—namely, the principle of natural selection in the struggle for existence. It was partly owing to his failure to detect this supremely important element, and partly to the poor condition of all biological science at the time, that Lamarck did not succeed in establishing more firmly his theory of the common descent of man and the other animals.
Independently of Lamarck, the older German school of natural philosophy, especially Reinhold Treviranus, in his Biologie (1802), and Lorentz Oken, in his Naturphilosophie (1809), turned its attention to the problem of evolution about the end of the eighteenth and beginning of the nineteenth century. I have described its work in my History of Creation (chap. iv). Here I can only deal with the brilliant genius whose evolutionary ideas are of special interest—the greatest of German poets, Wolfgang Goethe. With his keen eye for the beauties of nature, and his profound insight into its life, Goethe was early attracted to the study of various natural sciences. It was the favourite occupation of his leisure hours throughout life. He gave particular and protracted attention to the theory of colours. But the most valuable of his scientific studies are those which relate to that “living, glorious, precious thing,” the organism. He made profound research into the science of structures or morphology (morphæ = forms). Here, with the aid of comparative anatomy, he obtained the most brilliant results, and went far in advance of his time. I may mention, in particular, his vertebral theory of the skull, his discovery of the pineal gland in man, his system of the metamorphosis of plants, etc. These morphological studies led Goethe on to research into the formation and modification of organic structures which we must count as the first germ of the science of evolution. He approaches so near to the theory of descent that we must regard him, after Lamarck, as one of its earliest founders. It is true that he never formulated a complete scientific theory of evolution, but we find a number of remarkable suggestions of it in his splendid miscellaneous essays on morphology. Some of them are really among the very basic ideas of the science of evolution. He says, for instance (1807): “When we compare plants and animals in their most rudimentary forms, it is almost impossible to distinguish between them. But we may say that the plants and animals, beginning with an almost inseparable closeness, gradually advance along two divergent lines, until the plant at last grows in the solid, enduring tree and the animal attains in man to the highest degree of mobility and freedom.” That Goethe was not merely speaking in a poetical, but in a literal genealogical, sense of this close affinity of organic forms is clear from other remarkable passages in which he treats of their variety in outward form and unity in internal structure. He believes that every living thing has arisen by the interaction of two opposing formative forces or impulses. The internal or “centripetal” force, the type or “impulse to specification,” seeks to maintain the constancy of the specific forms in the succession of generations: this is heredity. The external or “centrifugal” force, the element of variation or “impulse to metamorphosis,” is continually modifying the species by changing their environment: this is adaptation. In these significant conceptions Goethe approaches very close to a recognition of the two great mechanical factors which we now assign as the chief causes of the formation of species.
However, in order to appreciate Goethe’s views on morphology, one must associate his decidedly monistic conception of nature with his pantheistic philosophy. The warm and keen interest with which he followed, in his last years, the controversies of contemporary French scientists, and especially the struggle between Cuvier and Geoffroy St. Hilaire (see chap. iv of The History of Creation), is very characteristic. It is also necessary to be familiar with his style and general tenour of thought in order to appreciate rightly the many allusions to evolution found in his writings. Otherwise, one is apt to make serious errors.
He approached so close, at the end of the eighteenth century, to the principles of the science of evolution that he may well be described as the first forerunner of Darwin, although he did not go so far as to formulate evolution as a scientific system, as Lamarck did.
We owe so much of the progress of scientific knowledge to Darwin’s Origin of Species that its influence is almost without parallel in the history of science. The literature of Darwinism grows from day to day, not only on the side of academic zoology and botany, the sciences which were chiefly affected by Darwin’s theory, but in a far wider circle, so that we find Darwinism discussed in popular literature with a vigour and zest that are given to no other scientific conception. This remarkable success is due chiefly to two circumstances. In the first place, all the sciences, and especially biology, have made astounding progress in the last half-century, and have furnished a very vast quantity of proofs of the theory of evolution. In striking contrast to the failure of Lamarck and the older scientists to attract attention to their effort to explain the origin of living things and of man, we have this second and successful effort of Darwin, which was able to gather to its support a large number of established facts. Availing himself of the progress already made, he had very different scientific proofs to allege than Lamarck, or St. Hilaire, or Goethe, or Treviranus had had. But, in the second place, we must acknowledge that Darwin had the special distinction of approaching the subject from an entirely new side, and of basing the theory of descent on a consistent system, which now goes by the name of Darwinism.
Lamarck had unsuccessfully attempted to explain the modification of organisms that descend from a common form chiefly by the action of habit and the use of organs, though with the aid of heredity. But Darwin’s success was complete when he independently sought to give a mechanical explanation, on a quite new ground, of this modification of plant and animal structures by adaptation and heredity. He was impelled to his theory of selection on the following grounds. He compared the origin of the various kinds of animals and plants which we modify artificially—by the action of artificial selection in horticulture and among domestic animals—with the origin of the species of animals and plants in their natural state. He then found that the agencies which we employ in the modification of forms by artificial selection are also at work in Nature. The chief of these agencies he held to be “the struggle for life.” The gist of this peculiarly Darwinian idea is given in this formula: The struggle for existence produces new species without premeditated design in the life of Nature, in the same way that the will of man consciously selects new races in artificial conditions. The gardener or the farmer selects new forms as he wills for his own profit, by ingeniously using the agency of heredity and adaptation for the modification of structures; so, in the natural state, the struggle for life is always unconsciously modifying the various species of living things. This struggle for life, or competition of organisms in securing the means of subsistence, acts without any conscious design, but it is none the less effective in modifying structures. As heredity and adaptation enter into the closest reciprocal action under its influence, new structures, or alterations of structure, are produced; and these are purposive in the sense that they serve the organism when formed, but they were produced without any pre-conceived aim.
This simple idea is the central thought of Darwinism, or the theory of selection. Darwin conceived this idea at an early date, and then, for more than twenty years, worked at the collection of empirical evidence in support of it before he published his theory. His grandfather, Erasmus Darwin, was an able scientist of the older school of natural philosophy, who published a number of natural-philosophic works about the end of the eighteenth century. The most important of them is his Zoonomia, published in 1794, in which he expounds views similar to those of Goethe and Lamarck, without really knowing anything of the work of these contemporaries. However, in the writings of the grandfather the plastic imagination rather outran the judgment, while in Charles Darwin the two were better balanced.
Darwin did not publish any account of his theory until 1858, when Alfred Russel Wallace, who had independently reached the same theory of selection, published his own work. In the following year appeared the Origin of Species, in which he develops it at length and supports it with a mass of proof. Wallace had reached the same conclusion, but he had not so clear a perception as Darwin of the effectiveness of natural selection in forming species, and did not develop the theory so fully. Nevertheless, Wallace’s writings, especially those on mimicry, etc., and an admirable work on The Geographical Distribution of Animals, contain many fine original contributions to the theory of selection. Unfortunately, this gifted scientist has since devoted himself to spiritism.[10]
[10] Darwin and Wallace arrived at the theory quite independently. Vide Wallace’s Contributions to the Theory of Natural Selection (1870) and Darwinism (1891).
Darwin’s Origin of Species had an extraordinary influence, though not at first on the experts of the science. It took zoologists and botanists several years to recover from the astonishment into which they had been thrown through the revolutionary idea of the work. But its influence on the special sciences with which we zoologists and botanists are concerned has increased from year to year; it has introduced a most healthy fermentation in every branch of biology, especially in comparative anatomy and ontogeny, and in zoological and botanical classification. In this way it has brought about almost a revolution in the prevailing views.
However, the point which chiefly concerns us here—the extension of the theory to man—was not touched at all in Darwin’s first work in 1859. It was believed for several years that he had no thought of applying his principles to man, but that he shared the current idea of man holding a special position in the universe. Not only ignorant laymen (especially several theologians), but also a number of men of science, said very naively that Darwinism in itself was not to be opposed; that it was quite right to use it to explain the origin of the various species of plants and animals, but that it was totally inapplicable to man.
In the meantime, however, it seemed to a good many thoughtful people, laymen as well as scientists, that this was wrong; that the descent of man from some other animal species, and immediately from some ape-like mammal, followed logically and necessarily from Darwin’s reformed theory of evolution. Many of the acuter opponents of the theory saw at once the justice of this position, and, as this consequence was intolerable, they wanted to get rid of the whole theory.
The first scientific application of the Darwinian theory to man was made by Huxley, the greatest zoologist in England. This able and learned scientist, to whom zoology owes much of its progress, published in 1863 a small work entitled Evidence as to Man’s Place in Nature. In the extremely important and interesting lectures which made up this work he proved clearly that the descent of man from the ape followed necessarily from the theory of descent. If that theory is true, we are bound to conceive the animals which most closely resemble man as those from which humanity has been gradually evolved. About the same time Carl Vogt published a larger work on the same subject. We must also mention Gustav Jaeger and Friedrich Rolle among the zoologists who accepted and taught the theory of evolution immediately after the publication of Darwin’s book, and maintained that the descent of man from the lower animals logically followed from it. The latter published, in 1866, a work on the origin and position of man.
About the same time I attempted, in the second volume of my General Morphology (1866), to apply the theory of evolution to the whole organic kingdom, including man.[11] I endeavoured to sketch the probable ancestral trees of the various classes of the animal world, the protists, and the plants, as it seemed necessary to do on Darwinian principles, and as we can actually do now with a high degree of confidence. If the theory of descent, which Lamarck first clearly formulated and Darwin thoroughly established, is true, we should be able to draw up a natural classification of plants and animals in the light of their genealogy, and to conceive the large and small divisions of the system as the branches and twigs of an ancestral tree. The eight genealogical tables which I inserted in the second volume of the General Morphology are the first sketches of their kind. In Chapter 27, particularly, I trace the chief stages in man’s ancestry, as far as it is possible to follow it through the vertebrate stem. I tried especially to determine, as well as one could at that time, the position of man in the classification of the mammals and its genealogical significance. I have greatly improved this attempt, and treated it in a more popular form, in chaps. xxvi–xxviii of my History of Creation (1868).[12]
[11] Huxley spoke of this “as one of the greatest scientific works ever published.”—Translator.
[12] Of which Darwin said that the Descent of Man would probably never have been written if he had seen it earlier.—Translator.
It was not until 1871, twelve years after the appearance of The Origin of Species, that Darwin published the famous work which made the much-contested application of his theory to man, and crowned the splendid structure of his system. This important work was The Descent of Man, and Selection in Relation to Sex. In this Darwin expressly drew the conclusion, with rigorous logic, that man also must have been developed out of lower species, and described the important part played by sexual selection in the elevation of man and the other higher animals. He showed that the careful selection which the sexes exercise on each other in regard to sexual relations and procreation, and the æsthetic feeling which the higher animals develop through this, are of the utmost importance in the progressive development of forms and the differentiation of the sexes. The males choosing the handsomest females in one class of animals, and the females choosing only the finest-looking males in another, the special features and the sexual characteristics are increasingly accentuated. In fact, some of the higher animals develop in this connection a finer taste and judgment than man himself. But, even as regards man, it is to this sexual selection that we owe the family-life, which is the chief foundation of civilisation. The rise of the human race is due for the most part to the advanced sexual selection which our ancestors exercised in choosing their mates.
Darwin accepted in the main the general outlines of man’s ancestral tree, as I gave it in the General Morphology and the History of Creation, and admitted that his studies led him to the same conclusion. That he did not at once apply the theory to man in his first work was a commendable piece of discretion; such a sequel was bound to excite the strongest opposition to the whole theory. The first thing to do was to establish it as regards the animal and plant worlds. The subsequent extension to man was bound to be made sooner or later.
It is important to understand this very clearly. If all living things come from a common root, man must be included in the general scheme of evolution. On the other hand, if the various species were separately created, man, too, must have been created, and not evolved. We have to choose between these two alternatives. This cannot be too frequently or too strongly emphasised. Either all the species of animals and plants are of supernatural origin—created, not evolved—and in that case man also is the outcome of a creative act, as religion teaches, or the different species have been evolved from a few common, simple ancestral forms, and in that case man is the highest fruit of the tree of evolution.
We may state this briefly in the following principle—The descent of man from the lower animals is a special deduction which inevitably follows from the general inductive law of the whole theory of evolution. In this principle we have a clear and plain statement of the matter. Evolution is in reality nothing but a great induction, which we are compelled to make by the comparative study of the most important facts of morphology and physiology. But we must draw our conclusion according to the laws of induction, and not attempt to determine scientific truths by direct measurement and mathematical calculation. In the study of living things we can scarcely ever directly and fully, and with mathematical accuracy, determine the nature of phenomena, as is done in the simpler study of the inorganic world—in chemistry, physics, mineralogy, and astronomy. In the latter, especially, we can always use the simplest and absolutely safest method—that of mathematical determination. But in biology this is quite impossible for various reasons; one very obvious reason being that most of the facts of the science are very complicated and much too intricate to allow a direct mathematical analysis. The greater part of the phenomena that biology deals with are complicated historical processes, which are related to a far-reaching past, and as a rule can only be approximately estimated. Hence we have to proceed by induction—that is to say, to draw general conclusions, stage by stage, and with proportionate confidence, from the accumulation of detailed observations. These inductive conclusions cannot command absolute confidence, like mathematical axioms; but they approach the truth, and gain increasing probability, in proportion as we extend the basis of observed facts on which we build. The importance of these inductive laws is not diminished from the circumstance that they are looked upon merely as temporary acquisitions of science, and may be improved to any extent in the progress of scientific knowledge. The same may be said of the attainments of many other sciences, such as geology or archeology. However much they may be altered and improved in detail in the course of time, these inductive truths may retain their substance unchanged.
Now, when we say that the theory of evolution in the sense of Lamarck and Darwin is an inductive law—in fact, the greatest of all biological inductions—we rely, in the first place, on the facts of paleontology. This science gives us some direct acquaintance with the historical phenomena of the changes of species. From the situations in which we find the fossils in the various strata of the earth we gather confidently, in the first place, that the living population of the earth has been gradually developed, as clearly as the earth’s crust itself; and that, in the second place, several different populations have succeeded each other in the various geological periods. Modern geology teaches that the formation of the earth has been gradual, and unbroken by any violent revolutions. And when we compare together the various kinds of animals and plants which succeed each other in the history of our planet, we find, in the first place, a constant and gradual increase in the number of species from the earliest times until the present day; and, in the second place, we notice that the forms in each great group of animals and plants also constantly improve as the ages advance. Thus, of the vertebrates there are at first only the lower fishes; then come the higher fishes, and later the amphibia. Still later appear the three higher classes of vertebrates—the reptiles, birds, and mammals, for the first time; only the lowest and least perfect forms of the mammals are found at first; and it is only at a very late period that placental mammals appear, and man belongs to the latest and youngest branch of these. Thus perfection of form increases as well as variety from the earliest to the latest stage. That is a fact of the greatest importance. It can only be explained by the theory of evolution, with which it is in perfect harmony. If the different groups of plants and animals do really descend from each other, we must expect to find this increase in their number and perfection under the influence of natural selection, just as the succession of fossils actually discloses it to us.
Comparative anatomy furnishes a second series of facts which are of great importance for the forming of our inductive law. This branch of morphology compares the adult structures of living things, and seeks in the great variety of organic forms the stable and simple law of organisation, or the common type or structure. Since Cuvier founded this science at the beginning of the nineteenth century it has been a favourite study of the most distinguished scientists. Even before Cuvier’s time Goethe had been greatly stimulated by it, and induced to take up the study of morphology. Comparative osteology, or the philosophic study and comparison of the bony skeleton of the vertebrates—one of its most interesting sections—especially fascinated him, and led him to form the theory of the skull which I mentioned before. Comparative anatomy shows that the internal structure of the animals of each stem and the plants of each class is the same in its essential features, however much they differ in external appearance. Thus man has so great a resemblance in the chief features of his internal organisation to the other mammals that no comparative anatomist has ever doubted that he belongs to this class. The whole internal structure of the human body, the arrangement of its various systems of organs, the distribution of the bones, muscles, blood-vessels, etc., and the whole structure of these organs in the larger and the finer scale, agree so closely with those of the other mammals (such as the apes, rodents, ungulates, cetacea, marsupials, etc.) that their external differences are of no account whatever. We learn further from comparative anatomy that the chief features of animal structure are so similar in the various classes (fifty to sixty in number altogether) that they may all be comprised in from eight to twelve great groups. But even in these groups, the stem-forms or animal types, certain organs (especially the alimentary canal) can be proved to have been originally the same for all. We can only explain by the theory of evolution this essential unity in internal structure of all these animal forms that differ so much in outward appearance. This wonderful fact can only be really understood and explained when we regard the internal resemblance as an inheritance from common-stem forms, and the external differences as the effect of adaptation to different environments.
In recognising this, comparative anatomy has itself advanced to a higher stage. Gegenbaur, the most distinguished of recent students of this science, says that with the theory of evolution a new period began in comparative anatomy, and that the theory in turn found a touch stone in the science. “Up to now there is no fact in comparative anatomy that is inconsistent with the theory of evolution; indeed, they all lead to it. In this way the theory receives back from the science all the service it rendered to its method.” Until then students had marvelled at the wonderful resemblance of living things in their inner structure without being able to explain it. We are now in a position to explain the causes of this, by showing that this remarkable agreement is the necessary consequence of the inheriting of common stem-forms; while the striking difference in outward appearance is a result of adaptation to changes of environment. Heredity and adaptation alone furnish the true explanation.
But one special part of comparative anatomy is of supreme interest and of the utmost philosophic importance in this connection. This is the science of rudimentary or useless organs; I have given it the name of “dysteleology” in view of its philosophic consequences. Nearly every organism (apart from the very lowest), and especially every highly-developed animal or plant, including man, has one or more organs which are of no use to the body itself, and have no share in its functions or vital aims. Thus we all have, in various parts of our frame, muscles which we never use, as, for instance, in the shell of the ear and adjoining parts. In most of the mammals, especially those with pointed ears, these internal and external ear-muscles are of great service in altering the shell of the ear, so as to catch the waves of sound as much as possible. But in the case of man and other short-eared mammals these muscles are useless, though they are still present. Our ancestors having long abandoned the use of them, we cannot work them at all to-day. In the inner corner of the eye we have a small crescent-shaped fold of skin; this is the last relic of a third inner eye-lid, called the nictitating (winking) membrane. This membrane is highly developed and of great service in some of our distant relations, such as fishes of the shark type and several other vertebrates; in us it is shrunken and useless. In the intestines we have a process that is not only quite useless, but may be very harmful—the vermiform appendage. This small intestinal appendage is often the cause of a fatal illness. If a cherry-stone or other hard body is unfortunately squeezed through its narrow aperture during digestion, a violent inflammation is set up, and often proves fatal. This appendix has no use whatever now in our frame; it is a dangerous relic of an organ that was much larger and was of great service in our vegetarian ancestors. It is still large and important in many vegetarian animals, such as apes and rodents.
There are similar rudimentary organs in all parts of our body, and in all the higher animals. They are among the most interesting phenomena to which comparative anatomy introduces us; partly because they furnish one of the clearest proofs of evolution, and partly because they most strikingly refute the teleology of certain philosophers. The theory of evolution enables us to give a very simple explanation of these phenomena.
We have to look on them as organs which have fallen into disuse in the course of many generations. With the decrease in the use of its function, the organ itself shrivels up gradually, and finally disappears. There is no other way of explaining rudimentary organs. Hence they are also of great interest in philosophy; they show clearly that the monistic or mechanical view of the organism is the only correct one, and that the dualistic or teleological conception is wrong. The ancient legend of the direct creation of man according to a pre-conceived plan and the empty phrases about “design” in the organism are completely shattered by them. It would be difficult to conceive a more thorough refutation of teleology than is furnished by the fact that all the higher animals have these rudimentary organs.
The theory of evolution finds its broadest inductive foundation in the natural classification of living things, which arranges all the various forms in larger and smaller groups, according to their degree of affinity. These groupings or categories of classification—the varieties, species, genera, families, orders, classes, etc.—show such constant features of coordination and subordination that we are bound to look on them as genealogical, and represent the whole system in the form of a branching tree. This is the genealogical tree of the variously related groups; their likeness in form is the expression of a real affinity. As it is impossible to explain in any other way the natural tree-like form of the system of organisms, we must regard it at once as a weighty proof of the truth of evolution. The careful construction of these genealogical trees is, therefore, not an amusement, but the chief task of modern classification.
Among the chief phenomena that bear witness to the inductive law of evolution we have the geographical distribution of the various species of animals and plants over the surface of the earth, and their topographical distribution on the summits of mountains and in the depths of the ocean. The scientific study of these features—the “science of distribution,” or chorology (chora = a place)—has been pursued with lively interest since the discoveries made by Alexander von Humboldt. Until Darwin’s time the work was confined to the determination of the facts of the science, and chiefly aimed at settling the spheres of distribution of the existing large and small groups of living things. It was impossible at that time to explain the causes of this remarkable distribution, or the reasons why one group is found only in one locality and another in a different place, and why there is this manifold distribution at all. Here, again, the theory of evolution has given us the solution of the problem. It furnishes the only possible explanation when it teaches that the various species and groups of species descend from common stem-forms, whose ever-branching offspring have gradually spread themselves by migration over the earth. For each group of species we must admit a “centre of production,” or common home; this is the original habitat in which the ancestral form was developed, and from which its descendants spread out in every direction. Several of these descendants became in their turn the stem-forms for new groups of species, and these also scattered themselves by active and passive migration, and so on. As each migrating organism found a different environment in its new home, and adapted itself to it, it was modified, and gave rise to new forms.
This very important branch of science that deals with active and passive migration was founded by Darwin, with the aid of the theory of evolution; and at the same time he advanced the true explanation of the remarkable relation or similarity of the living population in any locality to the fossil forms found in it. Moritz Wagner very ably developed his idea under the title of “the theory of migration.” In my opinion, this famous traveller has rather over-estimated the value of his theory of migration when he takes it to be an indispensable condition of the formation of new species and opposes the theory of selection. The two theories are not opposed in their main features. Migration (by which the stem-form of a new species is isolated) is really only a special case of selection. The striking and interesting facts of chorology can be explained only by the theory of evolution, and therefore we must count them among the most important of its inductive bases.
The same must be said of all the remarkable phenomena which we perceive in the economy of the living organism. The many and various relations of plants and animals to each other and to their environment, which are treated in bionomy (from nomos, law or norm, and bios, life), the interesting facts of parasitism, domesticity, care of the young, social habits, etc., can only be explained by the action of heredity and adaptation. Formerly people saw only the guidance of a beneficent Providence in these phenomena; to-day we discover in them admirable proofs of the theory of evolution. It is impossible to understand them except in the light of this theory and the struggle for life.
Finally, we must, in my opinion, count among the chief inductive bases of the theory of evolution the fœtal development of the individual organism, the whole science of embryology or ontogeny. But as the later chapters will deal with this in detail, I need say nothing further here. I shall endeavour in the following pages to show, step by step, how the whole of the embryonic phenomena form a massive chain of proof for the theory of evolution; for they can be explained in no other way. In thus appealing to the close causal connection between ontogenesis and phylogenesis, and taking our stand throughout on the biogenetic law, we shall be able to prove, stage by stage, from the facts of embryology, the evolution of man from the lower animals.
The general adoption of the theory of evolution has definitely closed the controversy as to the nature or definition of the species. The word has no absolute meaning whatever, but is only a group-name, or category of classification, with a purely relative value. In 1857, it is true, a famous and gifted, but inaccurate and dogmatic, scientist, Louis Agassiz, attempted to give an absolute value to these “categories of classification.” He did this in his Essay on Classification, in which he turns upside down the phenomena of organic nature, and, instead of tracing them to their natural causes, examines them through a theological prism. The true species (bona species) was, he said, an “incarnate idea of the Creator.” Unfortunately, this pretty phrase has no more scientific value than all the other attempts to save the absolute or intrinsic value of the species.
The dogma of the fixity and creation of species lost its last great champion when Agassiz died in 1873. The opposite theory, that all the different species descend from common stem-forms, encounters no serious difficulty to-day. All the endless research into the nature of the species, and the possibility of several species descending from a common ancestor, has been closed to-day by the removal of the sharp limits that had been set up between species and varieties on the one hand, and species and genera on the other. I gave an analytic proof of this in my monograph on the sponges (1872), having made a very close study of variability in this small but highly instructive group, and shown the impossibility of making any dogmatic distinction of species. According as the classifier takes his ideas of genus, species, and variety in a broader or in a narrower sense, he will find in the small group of the sponges either one genus with three species, or three genera with 238 species, or 113 genera with 591 species. Moreover, all these forms are so connected by intermediate forms that we can convincingly prove the descent of all the sponges from a common stem-form, the olynthus.
Here, I think, I have given an analytic solution of the problem of the origin of species, and so met the demand of certain opponents of evolution for an actual instance of descent from a stem-form. Those who are not satisfied with the synthetic proofs of the theory of evolution which are provided by comparative anatomy, embryology, paleontology, dysteleology, chorology, and classification, may try to refute the analytic proof given in my treatise on the sponge, the outcome of five years of assiduous study. I repeat: It is now impossible to oppose evolution on the ground that we have no convincing example of the descent of all the species of a group from a common ancestor. The monograph on the sponges furnishes such a proof, and, in my opinion, an indisputable proof. Any man of science who will follow the protracted steps of my inquiry and test my assertions will find that in the case of the sponges we can follow the actual evolution of species in a concrete case. And if this is so, if we can show the origin of all the species from a common form in one single class, we have the solution of the problem of man’s origin, because we are in a position to prove clearly his descent from the lower animals.
At the same time, we can now reply to the often-repeated assertion, even heard from scientists of our own day, that the descent of man from the lower animals, and proximately from the apes, still needs to be “proved with certainty.” These “certain proofs” have been available for a long time; one has only to open one’s eyes to see them. It is a mistake to seek them in the discovery of intermediate forms between man and the ape, or the conversion of an ape into a human being by skilful education. The proofs lie in the great mass of empirical material we have already collected. They are furnished in the strongest form by the data of comparative anatomy and embryology, completed by paleontology. It is not a question now of detecting new proofs of the evolution of man, but of examining and understanding the proofs we already have.
I was almost alone thirty-six years ago when I made the first attempt, in my General Morphology, to put organic science on a mechanical foundation through Darwin’s theory of descent. The association of ontogeny and phylogeny and the proof of the intimate causal connection between these two sections of the science of evolution, which I expounded in my work, met with the most spirited opposition on nearly all sides. The next ten years were a terrible “struggle for life” for the new theory. But for the last twenty-five years the tables have been turned. The phylogenetic method has met with so general a reception, and found so prolific a use in every branch of biology, that it seems superfluous to treat any further here of its validity and results. The proof of it lies in the whole morphological literature of the last three decades. But no other science has been so profoundly modified in its leading thoughts by this adoption, and been forced to yield such far-reaching consequences, as that science which I am now seeking to establish—monistic anthropogeny.
This statement may seem to be rather audacious, since the very next branch of biology, anthropology in the stricter sense, makes very little use of these results of anthropogeny, and sometimes expressly opposes them.[13] This applies especially to the attitude which has characterised the German Anthropological Society (the Deutsche Gesellschaft fur Anthropologie) for some thirty years. Its powerful president, the famous pathologist, Rudolph Virchow, is chiefly responsible for this. Until his death (September 5th, 1902) he never ceased to reject the theory of descent as unproven, and to ridicule its chief consequence—the descent of man from a series of mammal ancestors—as a fantastic dream. I need only recall his well-known expression at the Anthropological Congress at Vienna in 1894, that “it would be just as well to say man came from the sheep or the elephant as from the ape.”
[13] This does not apply to English anthropologists, who are almost all evolutionists.
Virchow’s assistant, the secretary of the German Anthropological Society, Professor Johannes Ranke of Munich, has also indefatigably opposed transformism: he has succeeded in writing a work in two volumes (Der Mensch), in which all the facts relating to his organisation are explained in a sense hostile to evolution. This work has had a wide circulation, owing to its admirable illustrations and its able treatment of the most interesting facts of anatomy and physiology—exclusive of the sexual organs! But, as it has done a great deal to spread erroneous views among the general public, I have included a criticism of it in my History of Creation, as well as met Virchow’s attacks on anthropogeny.
Neither Virchow, nor Ranke, nor any other “exact” anthropologist, has attempted to give any other natural explanation of the origin of man. They have either set completely aside this “question of questions” as a transcendental problem, or they have appealed to religion for its solution. We have to show that this rejection of the rational explanation is totally without justification. The fund of knowledge which has accumulated in the progress of biology in the nineteenth century is quite adequate to furnish a rational explanation, and to establish the theory of the evolution of man on the solid facts of his embryology.
In order to understand clearly the course of human embryology, we must select the more important of its wonderful and manifold processes for fuller explanation, and then proceed from these to the innumerable features of less importance. The most important feature in this sense, and the best starting-point for ontogenetic study, is the fact that man is developed from an ovum, and that this ovum is a simple cell. The human ovum does not materially differ in form and composition from that of the other mammals, whereas there is a distinct difference between the fertilised ovum of the mammal and that of any other animal.
Fig. 1—The human ovum. The globular mass of yelk (b) is enclosed by a transparent membrane (the ovolemma or zona pellucida [a]), and contains a noncentral nucleus (the germinal vesicle, c). Cf. Fig. 14.
This fact is so important that few should be unaware of its extreme significance; yet it was quite unknown in the first quarter of the nineteenth century. As we have seen, the human and mammal ovum was not discovered until 1827, when Carl Ernst von Baer detected it. Up to that time the larger vesicles, in which the real and much smaller ovum is contained, had been wrongly regarded as ova. The important circumstance that this mammal ovum is a simple cell, like the ovum of other animals, could not, of course, be recognised until the cell theory was established. This was not done, by Schleiden for the plant and Schwann for the animal, until 1838. As we have seen, this cell theory is of the greatest service in explaining the human frame and its embryonic development. Hence we must say a few words about the actual condition of the theory and the significance of the views it has suggested.
In order properly to appreciate the cellular theory, the most important element in our science, it is necessary to understand in the first place that the cell is a unified organism, a self-contained living being. When we anatomically dissect the fully-formed animal or plant into its various organs, and then examine the finer structure of these organs with the microscope, we are surprised to find that all these different parts are ultimately made up of the same structural element or unit. This common unit of structure is the cell. It does not matter whether we thus dissect a leaf, flower, or fruit, or a bone, muscle, gland, or bit of skin, etc.; we find in every case the same ultimate constituent, which has been called the cell since Schleiden’s discovery. There are many opinions as to its real nature, but the essential point in our view of the cell is to look upon it as a self-contained or independent living unit. It is, in the words of Brucke, “an elementary organism.” We may define it most precisely as the ultimate organic unit, and, as the cells are the sole active principles in every vital function, we may call them the “plastids,” or “formative elements.” This unity is found in both the anatomic structure and the physiological function. In the case of the protists, the entire organism usually consists of a single independent cell throughout life. But in the tissue-forming animals and plants, which are the great majority, the organism begins its career as a simple cell, and then grows into a cell-community, or, more correctly, an organised cell-state. Our own body is not really the simple unity that it is generally supposed to be. On the contrary, it is a very elaborate social system of countless microscopic organisms, a colony or commonwealth, made up of innumerable independent units, or very different tissue-cells.
In reality, the term “cell,” which existed long before the cell theory was formulated, is not happily chosen. Schleiden, who first brought it into scientific use in the sense of the cell theory, gave this name to the elementary organisms because, when you find them in the dissected plant, they generally have the appearance of chambers, like the cells in a bee-hive, with firm walls and a fluid or pulpy content. But some cells, especially young ones, are entirely without the enveloping membrane, or stiff wall. Hence we now generally describe the cell as a living, viscous particle of protoplasm, enclosing a firmer nucleus in its albuminoid body. There may be an enclosing membrane, as there actually is in the case of most of the plants; but it may be wholly lacking, as is the case with most of the animals. There is no membrane at all in the first stage. The young cells are usually round, but they vary much in shape later on. Illustrations of this will be found in the cells of the various parts of the body shown in Figs. 3–7.
Hence the essential point in the modern idea of the cell is that it is made up of two different active constituents—an inner and an outer part. The smaller and inner part is the nucleus (or caryon or cytoblastus, Fig. 1c and Fig. 2k). The outer and larger part, which encloses the other, is the body of the cell (celleus, cytos, or cytosoma). The soft living substance of which the two are composed has a peculiar chemical composition, and belongs to the group of the albuminoid plasma-substances (“formative matter”), or protoplasm. The essential and indispensable element of the nucleus is called nuclein (or caryoplasm); that of the cell body is called plastin (or cytoplasm). In the most rudimentary cases both substances seem to be quite simple and homogeneous, without any visible structure. But, as a rule, when we examine them under a high power of the microscope, we find a certain structure in the protoplasm. The chief and most common form of this is the fibrous or net-like “thready structure” (Frommann) and the frothy “honeycomb structure” (Bütschli).

Fig. 2—Stem-cell of one of the echinoderms (cytula, or “first segmentation-cell” = fertilised ovum), after Hertwig. k is the nucleus or caryon.
The shape or outer form of the cell is infinitely varied, in accordance with its endless power of adapting itself to the most diverse activities or environments. In its simplest form the cell is globular (Fig. 2). This normal round form is especially found in cells of the simplest construction, and those that are developed in a free fluid without any external pressure. In such cases the nucleus also is not infrequently round, and located in the centre of the cell-body (Fig. 2k). In other cases, the cells have no definite shape; they are constantly changing their form owing to their automatic movements. This is the case with the amœbæ (Fig. 15 and 16) and the amœboid travelling cells (Fig. 11), and also with very young ova (Fig. 13).However, as a rule, the cell assumes a definite form in the course of its career. In the tissues of the multicellular organism, in which a number of similar cells are bound together in virtue of certain laws of heredity, the shape is determined partly by the form of their connection and partly by their special functions. Thus, for instance, we find in the mucous lining of our tongue very thin and delicate flat cells of roundish shape (Fig. 3). In the outer skin we find similar, but harder, covering cells, joined together by saw-like edges (Fig. 4). In the liver and other glands there are thicker and softer cells, linked together in rows (Fig. 5).
The last-named tissues (Figs. 3–5) belong to the simplest and most primitive type, the group of the “covering-tissues,” or epithelia. In these “primary tissues” (to which the germinal layers belong) simple cells of the same kind are arranged in layers. The arrangement and shape are more complicated in the “secondary tissues,” which are gradually developed out of the primary, as in the tissues of the muscles, nerves, bones, etc. In the bones, for instance, which belong to the group of supporting or connecting organs, the cells (Fig. 6) are star-shaped, and are joined together by numbers of net-like interlacing processes; so, also, in the tissues of the teeth (Fig. 7), and in other forms of supporting-tissue, in which a soft or hard substance (intercellular matter, or base) is inserted between the cells.
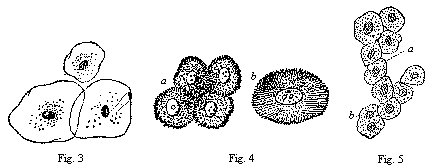
Fig. 3—Three epithelial cells from the mucous
lining of the tongue.
Fig. 4—Five spiny or grooved cells, with edges joined, from the
outer skin (epidermis): one of them (b) is isolated.
Fig. 5—Ten liver-cells: one of them (b) has two nuclei.
The cells also differ very much in size. The great majority of them are invisible to the naked eye, and can be seen only through the microscope (being as a rule between 1/2500 and 1/250 inch in diameter). There are many of the smaller plastids—such as the famous bacteria—which only come into view with a very high magnifying power. On the other hand, many cells attain a considerable size, and run occasionally to several inches in diameter, as do certain kinds of rhizopods among the unicellular protists (such as the radiolaria and thalamophora). Among the tissue-cells of the animal body many of the muscular fibres and nerve fibres are more than four inches, and sometimes more than a yard, in length. Among the largest cells are the yelk-filled ova; as, for instance, the yellow “yolk” in the hen’s egg, which we shall describe later (Fig. 15).
Cells also vary considerably in structure. In this connection we must first distinguish between the active and passive components of the cell. It is only the former, or active parts of the cell, that really live, and effect that marvellous world of phenomena to which we give the name of “organic life.” The first of these is the inner nucleus (caryoplasm), and the second the body of the cell (cytoplasm). The passive portions come third; these are subsequently formed from the others, and I have given them the name of “plasma-products.” They are partly external (cell-membranes and intercellular matter) and partly internal (cell-sap and cell-contents).
The nucleus (or caryon), which is usually of a simple roundish form, is quite structureless at first (especially in very young cells), and composed of homogeneous nuclear matter or caryoplasm (Fig. 2k). But, as a rule, it forms a sort of vesicle later on, in which we can distinguish a more solid nuclear base (caryobasis) and a softer or fluid nuclear sap (caryolymph). In a mesh of the nuclear network (or it may be on the inner side of the nuclear envelope) there is, as a rule, a dark, very opaque, solid body, called the nucleolus. Many of the nuclei contain several of these nucleoli (as, for instance, the germinal vesicle of the ova of fishes and amphibia). Recently a very small, but particularly important, part of the nucleus has been distinguished as the central body (centrosoma)—a tiny particle that is originally found in the nucleus itself, but is usually outside it, in the cytoplasm; as a rule, fine threads stream out from it in the cytoplasm. From the position of the central body with regard to the other parts it seems probable that it has a high physiological importance as a centre of movement; but it is lacking in many cells.
The cell-body also consists originally, and in its simplest form, of a homogeneous viscid plasmic matter. But, as a rule, only the smaller part of it is formed of the living active cell-substance (protoplasm); the greater part consists of dead, passive plasma-products (metaplasm). It is useful to distinguish between the inner and outer of these. External plasma-products (which are thrust out from the protoplasm as solid “structural matter”) are the cell-membranes and the intercellular matter. The internal plasma-products are either the fluid cell-sap or hard structures. As a rule, in mature and differentiated cells these various parts are so arranged that the protoplasm (like the caryoplasm in the round nucleus) forms a sort of skeleton or framework. The spaces of this network are filled partly with the fluid cell-sap and partly by hard structural products.
The simple round ovum, which we take as the starting-point of our study (Figs. 1 and 2), has in many cases the vague, indifferent features of the typical primitive cell. As a contrast to it, and as an instance of a very highly differentiated plastid, we may consider for a moment a large nerve-cell, or ganglionic cell, from the brain. The ovum stands potentially for the entire organism—in other words, it has the faculty of building up out of itself the whole multicellular body. It is the common parent of all the countless generations of cells which form the different tissues of the body; it unites all their powers in itself, though only potentially or in germ. In complete contrast to this, the neural cell in the brain (Fig. 9) develops along one rigid line. It cannot, like the ovum, beget endless generations of cells, of which some will become skin-cells, others muscle-cells, and others again bone-cells. But, on the other hand, the nerve-cell has become fitted to discharge the highest functions of life; it has the powers of sensation, will, and thought. It is a real soul-cell, or an elementary organ of the psychic activity. It has, therefore, a most elaborate and delicate structure.
Numbers of extremely fine threads, like the electric wires at a large telegraphic centre, cross and recross in the delicate protoplasm of the nerve cell, and pass out in the branching processes which proceed from it and put it in communication with other nerve-cells or nerve-fibres (a, b). We can only partly follow their intricate paths in the fine matter of the body of the cell.
Here we have a most elaborate apparatus, the delicate structure of which we are just beginning to appreciate through our most powerful microscopes, but whose significance is rather a matter of conjecture than knowledge. Its intricate structure corresponds to the very complicated functions of the mind. Nevertheless, this elementary organ of psychic activity—of which there are thousands in our brain—is nothing but a single cell. Our whole mental life is only the joint result of the combined activity of all these nerve-cells, or soul-cells. In the centre of each cell there is a large transparent nucleus, containing a small and dark nuclear body. Here, as elsewhere, it is the nucleus that determines the individuality of the cell; it proves that the whole structure, in spite of its intricate composition, amounts to only a single cell.

Fig. 8—Unfertilised ovum of an echinoderm (from Hertwig). The vesicular nucleus (or “germinal vesicle”) is globular, half the size of the round ovum, and encloses a nuclear framework, in the central knot of which there is a dark nucleolus (the “germinal spot”).
In contrast with this very elaborate and very strictly differentiated psychic cell (Fig. 9), we have our ovum (Figs. 1 and 2), which has hardly any structure at all. But even in the case of the ovum we must infer from its properties that its protoplasmic body has a very complicated chemical composition and a fine molecular structure which escapes our observation. This presumed molecular structure of the plasm is now generally admitted; but it has never been seen, and, indeed, lies far beyond the range of microscopic vision. It must not be confused—as is often done—with the structure of the plasm (the fibrous network, groups of granules, honey-comb, etc.) which does come within the range of the microscope.
But when we speak of the cells as the elementary organisms, or structural units, or “ultimate individualities,” we must bear in mind a certain restriction of the phrases. I mean, that the cells are not, as is often supposed, the very lowest stage of organic individuality. There are yet more elementary organisms to which I must refer occasionally. These are what we call the “cytodes” (cytos = cell), certain living, independent beings, consisting only of a particle of plasson—an albuminoid substance, which is not yet differentiated into caryoplasm and cytoplasm, but combines the properties of both. Those remarkable beings called the monera—especially the chromacea and bacteria—are specimens of these simple cytodes. (Compare Chapter XIX.) To be quite accurate, then, we must say: the elementary organism, or the ultimate individual, is found in two different stages. The first and lower stage is the cytode, which consists merely of a particle of plasson, or quite simple plasm. The second and higher stage is the cell, which is already divided or differentiated into nuclear matter and cellular matter. We comprise both kinds—the cytodes and the cells—under the name of plastids (“formative particles”), because they are the real builders of the organism. However, these cytodes are not found, as a rule, in the higher animals and plants; here we have only real cells with a nucleus. Hence, in these tissue-forming organisms (both plant and animal) the organic unit always consists of two chemically and anatomically different parts—the outer cell-body and the inner nucleus.
In order to convince oneself that this cell is really an independent organism, we have only to observe the development and vital phenomena of one of them. We see then that it performs all the essential functions of life—both vegetal and animal—which we find in the entire organism. Each of these tiny beings grows and nourishes itself independently. It takes its food from the surrounding fluid; sometimes, even, the naked cells take in solid particles at certain points of their surface—in other words, “eat” them—without needing any special mouth and stomach for the purpose (cf. Fig. 19).
Further, each cell is able to reproduce itself. This multiplication, in most cases, takes the form of a simple cleavage, sometimes direct, sometimes indirect; the simple direct (or “amitotic”) division is less common, and is found, for instance, in the blood cells (Fig. 10). In these the nucleus first divides into two equal parts by constriction. The indirect (or “mitotic”) cleavage is much more frequent; in this the caryoplasm of the nucleus and the cytoplasm of the cell-body act upon each other in a peculiar way, with a partial dissolution (caryolysis), the formation of knots and loops (mitosis), and a movement of the halved plasma-particles towards two mutually repulsive poles of attraction (caryokinesis, Fig. 11.)
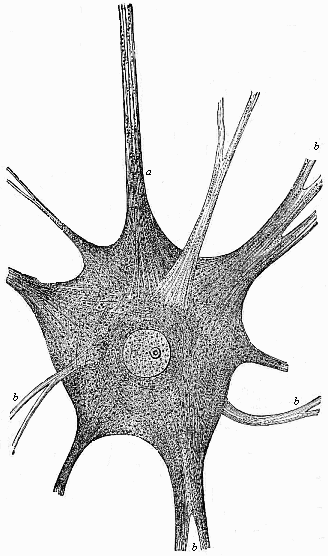
Fig. 9—A large branching nerve-cell, or “soul-cell”, from the brain of an electric fish (Torpedo). In the middle of the cell is the large transparent round nucleus, one nucleolus, and, within the latter again, a nucleolinus. The protoplasm of the cell is split into innumerable fine threads (or fibrils), which are embedded in intercellular matter, and are prolonged into the branching processes of the cell (b). One branch (a) passes into a nerve-fibre. (From Max Schultze.)

Fig. 10—Blood-cells, multiplying by direct division, from the blood of the embryo of a stag. Originally, each blood-cell has a nucleus and is round (a). When it is going to multiply, the nucleus divides into two (b, c, d). Then the protoplasmic body is constricted between the two nuclei, and these move away from each other (e). Finally, the constriction is complete, and the cell splits into two daughter-cells (f). (From Frey.)
The intricate physiological processes which accompany this “mitosis” have been very closely studied of late years. The inquiry has led to the detection of certain laws of evolution which are of extreme importance in connection with heredity. As a rule, two very different parts of the nucleus play an important part in these changes. They are: the chromatin, or coloured nuclear substance, which has a peculiar property of tingeing itself deeply with certain colouring matters (carmine, hæmatoxylin, etc.), and the achromin (or linin, or achromatin), a colourless nuclear substance that lacks this property. The latter generally forms in the dividing cell a sort of spindle, at the poles of which there is a very small particle, also colourless, called the “central body” (centrosoma). This acts as the centre or focus in a “sphere of attraction” for the granules of protoplasm in the surrounding cell-body, and assumes a star-like appearance (the cell-star, or monaster). The two central bodies, standing opposed to each other at the poles of the nuclear spindle, form “the double-star” (or amphiaster, Fig. 11, B C). The chromatin often forms a long, irregularly-wound thread—“the coil” (spirema, Fig. A). At the commencement of the cleavage it gathers at the equator of the cell, between the stellar poles, and forms a crown of U-shaped loops (generally four or eight, or some other definite number). The loops split lengthwise into two halves (B), and these back away from each other towards the poles of the spindle (C). Here each group forms a crown once more, and this, with the corresponding half of the divided spindle, forms a fresh nucleus (D). Then the protoplasm of the cell-body begins to contract in the middle, and gather about the new daughter-nuclei, and at last the two daughter-cells become independent beings.
Between this common mitosis, or indirect cell-division—which is the normal cleavage-process in most cells of the higher animals and plants—and the simple direct division (Fig. 10) we find every grade of segmentation; in some circumstances even one kind of division may be converted into another.
The plastid is also endowed with the functions of movement and sensation. The single cell can move and creep about, when it has space for free movement and is not prevented by a hard envelope; it then thrusts out at its surface processes like fingers, and quickly withdraws them again, and thus changes its shape (Fig. 12). Finally, the young cell is sensitive, or more or less responsive to stimuli; it makes certain movements on the application of chemical and mechanical irritation. Hence we can ascribe to the individual cell all the chief functions which we comprehend under the general heading of “life”—sensation, movement, nutrition, and reproduction. All these properties of the multicellular and highly developed animal are also found in the single animal-cell, at least in its younger stages. There is no longer any doubt about this, and so we may regard it as a solid and important base of our physiological conception of the elementary organism.
Without going any further here into these very interesting phenomena of the life of the cell, we will pass on to consider the application of the cell theory to the ovum. Here comparative research yields the important result that every ovum is at first a simple cell. I say this is very important, because our whole science of embryology now resolves itself into the problem: “How does the multicellular organism arise from the unicellular?” Every organic individual is at first a simple cell, and as such an elementary organism, or a unit of individuality. This cell produces a cluster of cells by segmentation, and from these develops the multicellular organism, or individual of higher rank.
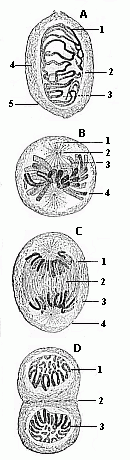
A. Mother-cell
(Knot, spirema)
1. Nuclear
threads (chromosomata) (coloured nuclear matter, chromatin)
2. Nuclear
membrane
3. Nuclear sap
4. Cytosoma
5. Protoplasm of the
cell-body
B. Mother-star, the loops beginning to split
lengthways (nuclear membrane gone)
1. Star-like appearance in
cytoplasm
2. Centrosoma (sphere of attraction)
3. Nuclear spindle
(achromin, colourless matter)
4. Nuclear loops (chromatin, coloured
matter)
C. The two daughter-stars,
produced
by the breaking of the loops of the mother-star (moving away)
1. Upper
daughter-crown
2. Connecting threads of the two crowns (achromin)
3.
Lower daughter-crown
4. Double-star (amphiaster)
D. The two daughter-cells,
produced by the complete division of the
two nuclear halves (cytosomata still connected at the equator) (Double-knot,
Dispirema)
1. Upper daughter-nucleus
2. Equatorial constriction of
the cell-body
3. Lower daughter-nucleus.
Fig. 11—Indirect or mitotic cell-division (with caryolysis and caryokinesis) from the skin of the larva of a salamander. (From Rabl.).
When we examine a little closer the original features of the ovum, we notice the extremely significant fact that in its first stage the ovum is just the same simple and indefinite structure in the case of man and all the animals (Fig. 13). We are unable to detect any material difference between them, either in outer shape or internal constitution. Later, though the ova remain unicellular, they differ in size and shape, enclose various kinds of yelk-particles, have different envelopes, and so on. But when we examine them at their birth, in the ovary of the female animal, we find them to be always of the same form in the first stages of their life. In the beginning each ovum is a very simple, roundish, naked, mobile cell, without a membrane; it consists merely of a particle of cytoplasm enclosing a nucleus (Fig. 13). Special names have been given to these parts of the ovum; the cell-body is called the yelk (vitellus), and the cell-nucleus the germinal vesicle. As a rule, the nucleus of the ovum is soft, and looks like a small pimple or vesicle. Inside it, as in many other cells, there is a nuclear skeleton or frame and a third, hard nuclear body (the nucleolus). In the ovum this is called the germinal spot. Finally, we find in many ova (but not in all) a still further point within the germinal spot, a “nucleolin,” which goes by the name of the germinal point. The latter parts (germinal spot and germinal point) have, apparently, a minor importance, in comparison with the other two (the yelk and germinal vesicle). In the yelk we must distinguish the active formative yelk (or protoplasm = first plasm) from the passive nutritive yelk (or deutoplasm = second plasm).
Fig. 12—Mobile cells from the inflamed eye of a frog (from the watery fluid of the eye, the humor aqueus). The naked cells creep freely about, by (like the amœba or rhizopods) protruding fine processes from the uncovered protoplasmic body. These bodies vary continually in number, shape, and size. The nucleus of these amœboid lymph-cells (“travelling cells,” or planocytes) is invisible, because concealed by the numbers of fine granules which are scattered in the protoplasm. (From Frey.)
In many of the lower animals (such as sponges, polyps, and medusæ) the naked ova retain their original simple appearance until impregnation. But in most animals they at once begin to change; the change consists partly in the formation of connections with the yelk, which serve to nourish the ovum, and partly of external membranes for their protection (the ovolemma, or prochorion). A membrane of this sort is formed in all the mammals in the course of the embryonic process. The little globule is surrounded by a thick capsule of glass-like transparency, the zona pellucida, or ovolemma pellucidum (Fig. 14). When we examine it closely under the microscope, we see very fine radial streaks in it, piercing the zona, which are really very narrow canals. The human ovum, whether fertilised or not, cannot be distinguished from that of most of the other mammals. It is nearly the same everywhere in form, size, and composition. When it is fully formed, it has a diameter of (on an average) about 1/120 of an inch. When the mammal ovum has been carefully isolated, and held against the light on a glass-plate, it may be seen as a fine point even with the naked eye. The ova of most of the higher mammals are about the same size. The diameter of the ovum is almost always between 1/250 to 1/125 inch. It has always the same globular shape; the same characteristic membrane; the same transparent germinal vesicle with its dark germinal spot. Even when we use the most powerful microscope with its highest power, we can detect no material difference between the ova of man, the ape, the dog, and so on. I do not mean to say that there are no differences between the ova of these different mammals. On the contrary, we are bound to assume that there are such, at least as regards chemical composition. Even the ova of different men must differ from each other; otherwise we should not have a different individual from each ovum. It is true that our crude and imperfect apparatus cannot detect these subtle individual differences, which are probably in the molecular structure. However, such a striking resemblance of their ova in form, so great as to seem to be a complete similarity, is a strong proof of the common parentage of man and the other mammals. From the common germ-form we infer a common stem-form. On the other hand, there are striking peculiarities by which we can easily distinguish the fertilised ovum of the mammal from the fertilised ovum of the birds, amphibia, fishes, and other vertebrates (see the close of Chap. XXIX).
The fertilised bird-ovum (Fig. 15) is notably different. It is true that in its earliest stage (Fig. 13 E) this ovum also is very like that of the mammal (Fig. 13 F). But afterwards, while still within the oviduct, it takes up a quantity of nourishment and works this into the familiar large yellow yelk. When we examine a very young ovum in the hen’s oviduct, we find it to be a simple, small, naked, amœboid cell, just like the young ova of other animals (Fig. 13). But it then grows to the size we are familiar with in the round yelk of the egg. The nucleus of the ovum, or the germinal vesicle, is thus pressed right to the surface of the globular ovum, and is embedded there in a small quantity of transparent matter, the so-called white yelk. This forms a round white spot, which is known as the “tread” (cicatricula) (Fig. 15 b). From the tread a thin column of the white yelk penetrates through the yellow yelk to the centre of the globular cell, where it swells into a small, central globule (wrongly called the yelk-cavity, or latebra, Fig. 15 d′). The yellow yelk-matter which surrounds this white yelk has the appearance in the egg (when boiled hard) of concentric layers (c). The yellow yelk is also enclosed in a delicate structureless membrane (the membrana vitellina, a).
Fig. 13—Ova of various animals, executing amœboid movements, magnified. All the ova are naked cells of varying shape. In the dark fine-grained protoplasm (yelk) is a large vesicular nucleus (the germinal vesicle), and in this is seen a nuclear body (the germinal spot), in which again we often see a germinal point. Figs. A1–A4 represent the ovum of a sponge (Leuculmis echinus) in four successive movements. B1–B8 are the ovum of a parasitic crab (Chondracanthus cornutus), in eight successive movements. (From Edward von Beneden.) C1–C5 show the ovum of the cat in various stages of movement (from Pflüger); Fig. D the ovum of a trout; E the ovum of a chicken; F a human ovum.
As the large yellow ovum of the bird attains a diameter of several inches in the bigger birds, and encloses round yelk-particles, there was formerly a reluctance to consider it as a simple cell. This was a mistake. Every animal that has only one cell-nucleus, every amœba, every gregarina, every infusorium, is unicellular, and remains unicellular whatever variety of matter it feeds on. So the ovum remains a simple cell, however much yellow yelk it afterwards accumulates within its protoplasm. It is, of course, different, with the bird’s egg when it has been fertilised. The ovum then consists of as many cells as there are nuclei in the tread. Hence, in the fertilised egg which we eat daily, the yellow yelk is already a multicellular body. Its tread is composed of several cells, and is now commonly called the germinal disc. We shall return to this discogastrula in Chap. IX.
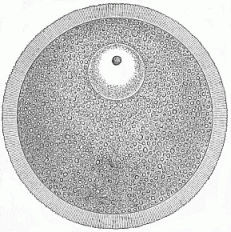
Fig. 14—The human ovum, taken from the female ovary, magnified. The whole ovum is a simple round cell. The chief part of the globular mass is formed by the nuclear yelk (deutoplasm), which is evenly distributed in the active protoplasm, and consists of numbers of fine yelk-granules. In the upper part of the yelk is the transparent round germinal vesicle, which corresponds to the nucleus. This encloses a darker granule, the germinal spot, which shows a nucleolus. The globular yelk is surrounded by the thick transparent germinal membrane (ovolemma, or zona pellucida). This is traversed by numbers of lines as fine as hairs, which are directed radially towards the centre of the ovum. These are called the pore-canals; it is through these that the moving spermatozoa penetrate into the yelk at impregnation.
When the mature bird-ovum has left the ovary and been fertilised in the oviduct, it covers itself with various membranes which are secreted from the wall of the oviduct. First, the large clear albuminous layer is deposited around the yellow yelk; afterwards, the hard external shell, with a fine inner skin. All these gradually forming envelopes and processes are of no importance in the formation of the embryo; they serve merely for the protection of the original simple ovum. We sometimes find extraordinarily large eggs with strong envelopes in the case of other animals, such as fishes of the shark type. Here, also, the ovum is originally of the same character as it is in the mammal; it is a perfectly simple and naked cell. But, as in the case of the bird, a considerable quantity of nutritive yelk is accumulated inside the original yelk as food for the developing embryo; and various coverings are formed round the egg. The ovum of many other animals has the same internal and external features. They have, however, only a physiological, not a morphological, importance; they have no direct influence on the formation of the fœtus. They are partly consumed as food by the embryo, and partly serve as protective envelopes. Hence we may leave them out of consideration altogether here, and restrict ourselves to material points—to the substantial identity of the original ovum in man and the rest of the animals (Fig. 13).
Now, let us for the first time make use of our biogenetic law; and directly apply this fundamental law of evolution to the human ovum. We reach a very simple, but very important, conclusion. From the fact that the human ovum and that of all other animals consists of a single cell, it follows immediately, according to the biogenetic law, that all the animals, including man, descend from a unicellular organism. If our biogenetic law is true, if the embryonic development is a summary or condensed recapitulation of the stem-history—and there can be no doubt about it—we are bound to conclude, from the fact that all the ova are at first simple cells, that all the multicellular organisms originally sprang from a unicellular being. And as the original ovum in man and all the other animals has the same simple and indefinite appearance, we may assume with some probability that this unicellular stem-form was the common ancestor of the whole animal world, including man. However, this last hypothesis does not seem to me as inevitable and as absolutely certain as our first conclusion.
Fig. 15—A fertilised ovum from the oviduct of a hen. The yellow yelk (c) consists of several concentric layers (d), and is enclosed in a thin yelk-membrane (a). The nucleus or germinal vesicle is seen above in the cicatrix or “tread” (b). From that point the white yelk penetrates to the central yelk-cavity (d′). The two kinds of yelk do not differ very much.
This inference from the unicellular embryonic form to the unicellular ancestor is so simple, but so important, that we cannot sufficiently emphasise it. We must, therefore, turn next to the question whether there are to-day any unicellular organisms, from the features of which we may draw some approximate conclusion as to the unicellular ancestors of the multicellular organisms. The answer is: Most certainly there are. There are assuredly still unicellular organisms which are, in their whole nature, really nothing more than permanent ova. There are independent unicellular organisms of the simplest character which develop no further, but reproduce themselves as such, without any further growth. We know to-day of a great number of these little beings, such as the gregarinæ, flagellata, acineta, infusoria, etc. However, there is one of them that has an especial interest for us, because it at once suggests itself when we raise our question, and it must be regarded as the unicellular being that approaches nearest to the real ancestral form. This organism is the Amœba.
Fig. 16—A creeping amœba (highly magnified). The whole organism is a simple naked cell, and moves about by means of the changing arms which it thrusts out of and withdraws into its protoplasmic body. Inside it is the roundish nucleus with its nucleolus.
For a long time now we have comprised under the general name of amœbæ a number of microscopic unicellular organisms, which are very widely distributed, especially in fresh-water, but also in the ocean; in fact, they have lately been discovered in damp soil. There are also parasitic amœbæ which live inside other animals. When we place one of these amœbæ in a drop of water under the microscope and examine it with a high power, it generally appears as a roundish particle of a very irregular and varying shape (Figs. 16 and 17). In its soft, slimy, semi-fluid substance, which consists of protoplasm, we see only the solid globular particle it contains, the nucleus. This unicellular body moves about continually, creeping in every direction on the glass on which we are examining it. The movement is effected by the shapeless body thrusting out finger-like processes at various parts of its surface; and these are slowly but continually changing, and drawing the rest of the body after them. After a time, perhaps, the action changes. The amœba suddenly stands still, withdraws its projections, and assumes a globular shape. In a little while, however, the round body begins to expand again, thrusts out arms in another direction, and moves on once more. These changeable processes are called “false feet,” or pseudopodia, because they act physiologically as feet, yet are not special organs in the anatomic sense. They disappear as quickly as they come, and are nothing more than temporary projections of the semi-fluid and structureless body.
Fig. 17—Division of a unicellular amœba (Amœba polypodia) in six stages. (From F. E. Schultze.) the dark spot is the nucleus, the lighter spot a contractile vacuole in the protoplasm. The latter reforms in one of the daughter-cells.)
If you touch one of these creeping amœbæ with a needle, or put a drop of acid in the water, the whole body at once contracts in consequence of this mechanical or physical stimulus. As a rule, the body then resumes its globular shape. In certain circumstances—for instance, if the impurity of the water lasts some time—the amœba begins to develop a covering. It exudes a membrane or capsule, which immediately hardens, and assumes the appearance of a round cell with a protective membrane. The amœba either takes its food directly by imbibition of matter floating in the water, or by pressing into its protoplasmic body solid particles with which it comes in contact. The latter process may be observed at any moment by forcing it to eat. If finely ground colouring matter, such as carmine or indigo, is put into the water, you can see the body of the amœba pressing these coloured particles into itself, the substance of the cell closing round them. The amœba can take in food in this way at any point on its surface, without having any special organs for intussusception and digestion, or a real mouth or gut.
The amœba grows by thus taking in food and dissolving the particles eaten in its protoplasm. When it reaches a certain size by this continual feeding, it begins to reproduce. This is done by the simple process of cleavage (Fig. 17). First, the nucleus divides into two parts. Then the protoplasm is separated between the two new nuclei, and the whole cell splits into two daughter-cells, the protoplasm gathering about each of the nuclei. The thin bridge of protoplasm which at first connects the daughter-cells soon breaks. Here we have the simple form of direct cleavage of the nuclei. Without mitosis, or formation of threads, the homogeneous nucleus divides into two halves. These move away from each other, and become centres of attraction for the enveloping matter, the protoplasm. The same direct cleavage of the nuclei is also witnessed in the reproduction of many other protists, while other unicellular organisms show the indirect division of the cell.
Hence, although the amœba is nothing but a simple cell, it is evidently able to accomplish all the functions of the multicellular organism. It moves, feels, nourishes itself, and reproduces. Some kinds of these amœbæ can be seen with the naked eye, but most of them are microscopically small. It is for the following reasons that we regard the amœbæ as the unicellular organisms which have special phylogenetic (or evolutionary) relations to the ovum. In many of the lower animals the ovum retains its original naked form until fertilisation, develops no membranes, and is then often indistinguishable from the ordinary amœba. Like the amœbæ, these naked ova may thrust out processes, and move about as travelling cells. In the sponges these mobile ova move about freely in the maternal body like independent amœbæ (Fig. 17). They had been observed by earlier scientists, but described as foreign bodies—namely, parasitic amœbæ, living parasitically on the body of the sponge. Later, however, it was discovered that they were not parasites, but the ova of the sponge. We also find this remarkable phenomenon among other animals, such as the graceful, bell-shaped zoophytes, which we call polyps and medusæ. Their ova remain naked cells, which thrust out amœboid projections, nourish themselves, and move about. When they have been fertilised, the multicellular organism is formed from them by repeated segmentation.
It is, therefore, no audacious hypothesis, but a perfectly sound conclusion, to regard the amœba as the particular unicellular organism which offers us an approximate illustration of the ancient common unicellular ancestor of all the metazoa, or multicellular animals. The simple naked amœba has a less definite and more original character than any other cell. Moreover, there is the fact that recent research has discovered such amœba-like cells everywhere in the mature body of the multicellular animals. They are found, for instance, in the human blood, side by side with the red corpuscles, as colourless blood-cells; and it is the same with all the vertebrates. They are also found in many of the invertebrates—for instance, in the blood of the snail. I showed, in 1859, that these colourless blood-cells can, like the independent amœbæ, take up solid particles, or “eat” (whence they are called phagocytes = “eating-cells,” Fig. 19). Lately, it has been discovered that many different cells may, if they have room enough, execute the same movements, creeping about and eating. They behave just like amœbæ (Fig. 12). It has also been shown that these “travelling-cells,” or planocytes, play an important part in man’s physiology and pathology (as means of transport for food, infectious matter, bacteria, etc.).
The power of the naked cell to execute these characteristic amœba-like movements comes from the contractility (or automatic mobility) of its protoplasm. This seems to be a universal property of young cells. When they are not enclosed by a firm membrane, or confined in a “cellular prison,” they can always accomplish these amœboid movements. This is true of the naked ova as well as of any other naked cells, of the “travelling-cells,” of various kinds in connective tissue, lymph-cells, mucus-cells, etc.
Fig. 18—Ovum of a sponge (Olynthus). The ovum creeps about in a body of the sponge by thrusting out ever-changing processes. It is indistinguishable from the common amœba.)
We have now, by our study of the ovum and the comparison of it with the amœba, provided a perfectly sound and most valuable foundation for both the embryology and the evolution of man. We have learned that the human ovum is a simple cell, that this ovum is not materially different from that of other mammals, and that we may infer from it the existence of a primitive unicellular ancestral form, with a substantial resemblance to the amœba.
The statement that the earliest progenitors of the human race were simple cells of this kind, and led an independent unicellular life like the amœba, has not only been ridiculed as the dream of a natural philosopher, but also been violently censured in theological journals as “shameful and immoral.” But, as I observed in my essay On the Origin and Ancestral Tree of the Human Race in 1870, this offended piety must equally protest against the “shameful and immoral” fact that each human individual is developed from a simple ovum, and that this human ovum is indistinguishable from those of the other mammals, and in its earliest stage is like a naked amœba. We can show this to be a fact any day with the microscope, and it is little use to close one’s eyes to “immoral” facts of this kind. It is as indisputable as the momentous conclusions we draw from it and as the vertebrate character of man (see Chap. XI).
Fig. 19—Blood-cells that eat, or phagocytes, from a naked sea-snail (Thetis), greatly magnified. I was the first to observe in the blood-cells of this snail the important fact that “the blood-cells of the invertebrates are unprotected pieces of plasm, and take in food, by means of their peculiar movements, like the amœbæ.” I had (in Naples, on May 10th, 1859) injected into the blood-vessels of one of these snails an infusion of water and ground indigo, and was greatly astonished to find the blood-cells themselves more or less filled with the particles of indigo after a few hours. After repeated injections I succeeded in “observing the very entrance of the coloured particles in the blood-cells, which took place just in the same way as with the amœba.” I have given further particulars about this in my Monograph on the Radiolaria.
We now see very clearly how extremely important the cell theory has been for our whole conception of organic nature. “Man’s place in nature” is settled beyond question by it. Apart from the cell theory, man is an insoluble enigma to us. Hence philosophers, and especially physiologists, should be thoroughly conversant with it. The soul of man can only be really understood in the light of the cell-soul, and we have the simplest form of this in the amœba. Only those who are acquainted with the simple psychic functions of the unicellular organisms and their gradual evolution in the series of lower animals can understand how the elaborate mind of the higher vertebrates, and especially of man, was gradually evolved from them. The academic psychologists who lack this zoological equipment are unable to do so.
This naturalistic and realistic conception is a stumbling-block to our modern idealistic metaphysicians and their theological colleagues. Fenced about with their transcendental and dualistic prejudices, they attack not only the monistic system we establish on our scientific knowledge, but even the plainest facts which go to form its foundation. An instructive instance of this was seen a few years ago, in the academic discourse delivered by a distinguished theologian, Willibald Beyschlag, at Halle, January 12th, 1900, on the occasion of the centenary festival. The theologian protested violently against the “materialistic dustmen of the scientific world who offer our people the diploma of a descent from the ape, and would prove to them that the genius of a Shakespeare or a Goethe is merely a distillation from a drop of primitive mucus.” Another well-known theologian protested against “the horrible idea that the greatest of men, Luther and Christ, were descended from a mere globule of protoplasm.” Nevertheless, not a single informed and impartial scientist doubts the fact that these greatest men were, like all other men—and all other vertebrates—developed from an impregnated ovum, and that this simple nucleated globule of protoplasm has the same chemical constitution in all the mammals.
The recognition of the fact that every man begins his individual existence as a simple cell is the solid foundation of all research into the genesis of man. From this fact we are forced, in virtue of our biogenetic law, to draw the weighty phylogenetic conclusion that the earliest ancestors of the human race were also unicellular organisms; and among these protozoa we may single out the vague form of the amœba as particularly important (cf. Chapter VI). That these unicellular ancestral forms did once exist follows directly from the phenomena which we perceive every day in the fertilised ovum. The development of the multicellular organism from the ovum, and the formation of the germinal layers and the tissues, follow the same laws in man and all the higher animals. It will, therefore, be our next task to consider more closely the impregnated ovum and the process of conception which produces it.
The process of impregnation or sexual conception is one of those phenomena that people love to conceal behind the mystic veil of supernatural power. We shall soon see, however, that it is a purely mechanical process, and can be reduced to familiar physiological functions. Moreover, this process of conception is of the same type, and is effected by the same organs, in man as in all the other mammals. The pairing of the male and female has in both cases for its main purpose the introduction of the ripe matter of the male seed or sperm into the female body, in the sexual canals of which it encounters the ovum. Conception then ensues by the blending of the two.
We must observe, first, that this important process is by no means so widely distributed in the animal and plant world as is commonly supposed. There is a very large number of lower organisms which propagate unsexually, or by monogamy; these are especially the sexless monera (chromacea, bacteria, etc.) but also many other protists, such as the amœbæ, foraminifera, radiolaria, myxomycetæ, etc. In these the multiplication of individuals takes place by unsexual reproduction, which takes the form of cleavage, budding, or spore-formation. The copulation of two coalescing cells, which in these cases often precedes the reproduction, cannot be regarded as a sexual act unless the two copulating plastids differ in size or structure. On the other hand, sexual reproduction is the general rule with all the higher organisms, both animal and plant; very rarely do we find asexual reproduction among them. There are, in particular, no cases of parthenogenesis (virginal conception) among the vertebrates.
Sexual reproduction offers an infinite variety of interesting forms in the different classes of animals and plants, especially as regards the mode of conception, and the conveyance of the spermatozoon to the ovum. These features are of great importance not only as regards conception itself, but for the development of the organic form, and especially for the differentiation of the sexes. There is a particularly curious correlation of plants and animals in this respect. The splendid studies of Charles Darwin and Hermann Müller on the fertilisation of flowers by insects have given us very interesting particulars of this.[14] This reciprocal service has given rise to a most intricate sexual apparatus. Equally elaborate structures have been developed in man and the higher animals, serving partly for the isolation of the sexual products on each side, partly for bringing them together in conception. But, however interesting these phenomena are in themselves, we cannot go into them here, as they have only a minor importance—if any at all—in the real process of conception. We must, however, try to get a very clear idea of this process and the meaning of sexual reproduction.
[14] See Darwin’s work, On the Various Contrivances by which Orchids are Fertilised (1862).
In every act of conception we have, as I said, to consider two different kinds of cells—a female and a male cell. The female cell of the animal organism is always called the ovum (or ovulum, egg, or egg-cell); the male cells are known as the sperm or seed-cells, or the spermatozoa (also spermium and zoospermium). The ripe ovum is, on the whole, one of the largest cells we know. It attains colossal dimensions when it absorbs great quantities of nutritive yelk, as is the case with birds and reptiles and many of the fishes. In the great majority of the animals the ripe ovum is rich in yelk and much larger than the other cells. On the other hand, the next cell which we have to consider in the process of conception, the male sperm-cell or spermatozoon, is one of the smallest cells in the animal body. Conception usually consists in the bringing into contact with the ovum of a slimy fluid secreted by the male, and this may take place either inside or out of the female body. This fluid is called sperm, or the male seed. Sperm, like saliva or blood, is not a simple fluid, but a thick agglomeration of innumerable cells, swimming about in a comparatively small quantity of fluid. It is not the fluid, but the independent male cells that swim in it, that cause conception.
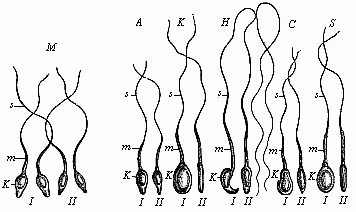
Fig. 20—Spermia or spermatozoa of various mammals. The pear-shaped flattened nucleus is seen from the front in I and sideways in II. k is the nucleus, m its middle part (protoplasm), s the mobile, serpent-like tail (or whip); M four human spermatozoa, A four spermatozoa from the ape; K from the rabbit; H from the mouse; C from the dog; S from the pig.
The spermatozoa of the great majority of animals have two characteristic features. Firstly, they are extraordinarily small, being usually the smallest cells in the body; and, secondly, they have, as a rule, a peculiarly lively motion, which is known as spermatozoic motion. The shape of the cell has a good deal to do with this motion. In most of the animals, and also in many of the lower plants (but not the higher) each of these spermatozoa has a very small, naked cell-body, enclosing an elongated nucleus, and a long thread hanging from it (Fig. 20). It was long before we could recognise that these structures are simple cells. They were formerly held to be special organisms, and were called “seed animals” (spermato-zoa, or spermato-zoidia); they are now scientifically known as spermia or spermidia, or as spermatosomata (seed-bodies) or spermatofila (seed threads). It took a good deal of comparative research to convince us that each of these spermatozoa is really a simple cell. They have the same shape as in many other vertebrates and most of the invertebrates. However, in many of the lower animals they have quite a different shape. Thus, for instance, in the craw fish they are large round cells, without any movement, equipped with stiff outgrowths like bristles (Fig. 21 f ). They have also a peculiar form in some of the worms, such as the thread-worms (filaria); in this case they are sometimes amœboid and like very small ova (Fig. 21 c to e). But in most of the lower animals (such as the sponges and polyps) they have the same pine-cone shape as in man and the other animals (Fig. 21 a, h).
Fig. 21—Spermatozoa or spermidia of various animals. (From Lang). a of a fish, b of a turbellaria worm (with two side-lashes), c to e of a nematode worm (amœboid spermatozoa), f from a craw fish (star-shaped), g from the salamander (with undulating membrane), h of an annelid (a and h are the usual shape).
When the Dutch naturalist Leeuwenhoek discovered these thread-like lively particles in 1677 in the male sperm, it was generally believed that they were special, independent, tiny animalcules, like the infusoria, and that the whole mature organism existed already, with all its parts, but very small and packed together, in each spermatozoon (see p.12). We now know that the mobile spermatozoa are nothing but simple and real cells, of the kind that we call “ciliated” (equipped with lashes, or cilia). In the previous illustrations we have distinguished in the spermatozoon a head, trunk, and tail. The “head” (Fig. 20 k) is merely the oval nucleus of the cell; the body or middle-part (m) is an accumulation of cell-matter; and the tail (s) is a thread-like prolongation of the same.
Moreover, we now know that these spermatozoa are not at all a peculiar form of cell; precisely similar cells are found in various other parts of the body. If they have many short threads projecting, they are called ciliated; if only one long, whip-shaped process (or, more rarely, two or four), caudate (tailed) cells.
Very careful recent examination of the spermia, under a very high microscopic power (Fig. 22 a, b), has detected some further details in the finer structure of the ciliated cell, and these are common to man and the anthropoid ape. The head (k) encloses the elliptic nucleus in a thin envelope of cytoplasm; it is a little flattened on one side, and thus looks rather pear-shaped from the front (b). In the central piece (m) we can distinguish a short neck and a longer connective piece (with central body). The tail consists of a long main section (h) and a short, very fine tail (e).
Fig. 22—A single human spermatozoon magnified; a shows it from the broader and b from the narrower side. k head (with nucleus), m middle-stem, h long-stem, and e tail. (From Retzius.)
The process of fertilisation by sexual conception consists, therefore, essentially in the coalescence and fusing together of two different cells. The lively spermatozoon travels towards the ovum by its serpentine movements, and bores its way into the female cell (Fig. 23). The nuclei of both sexual cells, attracted by a certain “affinity,” approach each other and melt into one.
The fertilised cell is quite another thing from the unfertilised cell. For if we must regard the spermia as real cells no less than the ova, and the process of conception as a coalescence of the two, we must consider the resultant cell as a quite new and independent organism. It bears in the cell and nuclear matter of the penetrating spermatozoon a part of the father’s body, and in the protoplasm and caryoplasm of the ovum a part of the mother’s body. This is clear from the fact that the child inherits many features from both parents. It inherits from the father by means of the spermatozoon, and from the mother by means of the ovum. The actual blending of the two cells produces a third cell, which is the germ of the child, or the new organism conceived. One may also say of this sexual coalescence that the stem-cell is a simple hermaphrodite; it unites both sexual substances in itself.
Fig. 23—The fertilisation of the ovum by the spermatozoon (of a mammal). One of the many thread-like, lively spermidia pierces through a fine pore-canal into the nuclear yelk. The nucleus of the ovum is invisible.
I think it necessary to emphasise the fundamental importance of this simple, but often unappreciated, feature in order to have a correct and clear idea of conception. With that end, I have given a special name to the new cell from which the child develops, and which is generally loosely called “the fertilised ovum,” or “the first segmentation sphere.” I call it “the stem-cell” (cytula). The name “stem-cell” seems to me the simplest and most suitable, because all the other cells of the body are derived from it, and because it is, in the strictest sense, the stem-father and stem-mother of all the countless generations of cells of which the multicellular organism is to be composed. That complicated molecular movement of the protoplasm which we call “life” is, naturally, something quite different in this stem-cell from what we find in the two parent-cells, from the coalescence of which it has issued. The life of the stem-cell or cytula is the product or resultant of the paternal life-movement that is conveyed in the spermatozoon and the maternal life-movement that is contributed by the ovum.
The admirable work done by recent observers has shown that the individual development, in man and the other animals, commences with the formation of a simple “stem-cell” of this character, and that this then passes, by repeated segmentation (or cleavage), into a cluster of cells, known as “the segmentation sphere” or “segmentation cells.” The process is most clearly observed in the ova of the echinoderms (star-fishes, sea-urchins, etc.). The investigations of Oscar and Richard Hertwig were chiefly directed to these. The main results may be summed up as follows:—
Conception is preceded by certain preliminary changes, which are very necessary—in fact, usually indispensable—for its occurrence. They are comprised under the general heading of “Changes prior to impregnation.” In these the original nucleus of the ovum, the germinal vesicle, is lost. Part of it is extruded, and part dissolved in the cell contents; only a very small part of it is left to form the basis of a fresh nucleus, the pronucleus femininus. It is the latter alone that combines in conception with the invading nucleus of the fertilising spermatozoon (the pronucleus masculinus).
The impregnation of the ovum commences with a decay of the germinal vesicle, or the original nucleus of the ovum (Fig. 8). We have seen that this is in most unripe ova a large, transparent, round vesicle. This germinal vesicle contains a viscous fluid (the caryolymph). The firm nuclear frame (caryobasis) is formed of the enveloping membrane and a mesh-work of nuclear threads running across the interior, which is filled with the nuclear sap. In a knot of the network is contained the dark, stiff, opaque nuclear corpuscle or nucleolus. When the impregnation of the ovum sets in, the greater part of the germinal vesicle is dissolved in the cell; the nuclear membrane and mesh-work disappear; the nuclear sap is distributed in the protoplasm; a small portion of the nuclear base is extruded; another small portion is left, and is converted into the secondary nucleus, or the female pro-nucleus (Fig. 24 e k).
The small portion of the nuclear base which is extruded from the impregnated ovum is known as the “directive bodies” or “polar cells”; there are many disputes as to their origin and significance, but we are as yet imperfectly acquainted with them. As a rule, they are two small round granules, of the same size and appearance as the remaining pro-nucleus. They are detached cell-buds; their separation from the large mother-cell takes place in the same way as in ordinary “indirect cell-division.” Hence, the polar cells are probably to be conceived as “abortive ova,” or “rudimentary ova,” which proceed from a simple original ovum by cleavage in the same way that several sperm-cells arise from one “sperm-mother-cell,” in reproduction from sperm. The male sperm-cells in the testicles must undergo similar changes in view of the coming impregnation as the ova in the female ovary. In this maturing of the sperm each of the original seed-cells divides by double segmentation into four daughter-cells, each furnished with a fourth of the original nuclear matter (the hereditary chromatin); and each of these four descendant cells becomes a spermatozoon, ready for impregnation. Thus is prevented the doubling of the chromatin in the coalescence of the two nuclei at conception. As the two polar cells are extruded and lost, and have no further part in the fertilisation of the ovum, we need not discuss them any further. But we must give more attention to the female pro-nucleus which alone remains after the extrusion of the polar cells and the dissolving of the germinal vesicle (Fig. 23 e k). This tiny round corpuscle of chromatin now acts as a centre of attraction for the invading spermatozoon in the large ripe ovum, and coalesces with its “head,” the male pro-nucleus. The product of this blending, which is the most important part of the act of impregnation, is the stem-nucleus, or the first segmentation nucleus (archicaryon)—that is to say, the nucleus of the new-born embryonic stem-cell or “first segmentation cell.” This stem-cell is the starting point of the subsequent embryonic processes.
Hertwig has shown that the tiny transparent ova of the echinoderms are the most convenient for following the details of this important process of impregnation. We can, in this case, easily and successfully accomplish artificial impregnation, and follow the formation of the stem-cell step by step within the space of ten minutes. If we put ripe ova of the star-fish or sea-urchin in a watch glass with sea-water and add a drop of ripe sperm-fluid, we find each ovum impregnated within five minutes. Thousands of the fine, mobile ciliated cells, which we have described as “sperm-threads” (Fig. 20), make their way to the ova, owing to a sort of chemical sensitive action which may be called “smell.” But only one of these innumerable spermatozoa is chosen—namely, the one that first reaches the ovum by the serpentine motions of its tail, and touches the ovum with its head. At the spot where the point of its head touches the surface of the ovum the protoplasm of the latter is raised in the form of a small wart, the “impregnation rise” (Fig. 25 A). The spermatozoon then bores its way into this with its head, the tail outside wriggling about all the time (Fig. 25 B, C). Presently the tail also disappears within the ovum. At the same time the ovum secretes a thin external yelk-membrane (Fig. 25 C), starting from the point of impregnation; and this prevents any more spermatozoa from entering.
Inside the impregnated ovum we now see a rapid series of most important changes. The pear-shaped head of the sperm-cell, or the “head of the spermatozoon,” grows larger and rounder, and is converted into the male pro-nucleus (Fig. 26 s k). This has an attractive influence on the fine granules or particles which are distributed in the protoplasm of the ovum; they arrange themselves in lines in the figure of a star. But the attraction or the “affinity” between the two nuclei is even stronger. They move towards each other inside the yelk with increasing speed, the male (Fig. 27 s k) going more quickly than the female nucleus (e k). The tiny male nucleus takes with it the radiating mantle which spreads like a star about it. At last the two sexual nuclei touch (usually in the centre of the globular ovum), lie close together, are flattened at the points of contact, and coalesce into a common mass. The small central particle of nuclein which is formed from this combination of the nuclei is the stem-nucleus, or the first segmentation nucleus; the new-formed cell, the product of the impregnation, is our stem-cell, or “first segmentation sphere” (Fig. 2).
Fig. 25—Impregnation of the ovum of a star-fish. (From Hertwig.) Only a small part of the surface of the ovum is shown. One of the numerous spermatozoa approaches the “impregnation rise” (A), touches it (B), and then penetrates into the protoplasm of the ovum (C).
Hence the one essential point in the process of sexual reproduction or impregnation is the formation of a new cell, the stem-cell, by the combination of two originally different cells, the female ovum and the male spermatozoon. This process is of the highest importance, and merits our closest attention; all that happens in the later development of this first cell and in the life of the organism that comes of it is determined from the first by the chemical and morphological composition of the stem-cell, its nucleus and its body. We must, therefore, make a very careful study of the rise and structure of the stem-cell.
The first question that arises is as to the two different active elements, the nucleus and the protoplasm, in the actual coalescence. It is obvious that the nucleus plays the more important part in this. Hence Hertwig puts his theory of conception in the principle: “Conception consists in the copulation of two cell-nuclei, which come from a male and a female cell.” And as the phenomenon of heredity is inseparably connected with the reproductive process, we may further conclude that these two copulating nuclei “convey the characteristics which are transmitted from parents to offspring.” In this sense I had in 1866 (in the ninth chapter of the General Morphology) ascribed to the reproductive nucleus the function of generation and heredity, and to the nutritive protoplasm the duties of nutrition and adaptation. As, moreover, there is a complete coalescence of the mutually attracted nuclear substances in conception, and the new nucleus formed (the stem-nucleus) is the real starting-point for the development of the fresh organism, the further conclusion may be drawn that the male nucleus conveys to the child the qualities of the father, and the female nucleus the features of the mother. We must not forget, however, that the protoplasmic bodies of the copulating cells also fuse together in the act of impregnation; the cell-body of the invading spermatozoon (the trunk and tail of the male ciliated cell) is dissolved in the yelk of the female ovum. This coalescence is not so important as that of the nuclei, but it must not be overlooked; and, though this process is not so well known to us, we see clearly at least the formation of the star-like figure (the radial arrangement of the particles in the plasma) in it (Figs. 26–27).
The older theories of impregnation generally went astray in regarding the large ovum as the sole base of the new organism, and only ascribed to the spermatozoon the work of stimulating and originating its development. The stimulus which it gave to the ovum was sometimes thought to be purely chemical, at other times rather physical (on the principle of transferred movement), or again a mystic and transcendental process. This error was partly due to the imperfect knowledge at that time of the facts of impregnation, and partly to the striking difference in the sizes of the two sexual cells. Most of the earlier observers thought that the spermatozoon did not penetrate into the ovum. And even when this had been demonstrated, the spermatozoon was believed to disappear in the ovum without leaving a trace. However, the splendid research made in the last three decades with the finer technical methods of our time has completely exposed the error of this. It has been shown that the tiny sperm-cell is not subordinated to, but coordinated with, the large ovum. The nuclei of the two cells, as the vehicles of the hereditary features of the parents, are of equal physiological importance. In some cases we have succeeded in proving that the mass of the active nuclear substance which combines in the copulation of the two sexual nuclei is originally the same for both.
These morphological facts are in perfect harmony with the familiar physiological truth that the child inherits from both parents, and that on the average they are equally distributed. I say “on the average,” because it is well known that a child may have a greater likeness to the father or to the mother; that goes without saying, as far as the primary sexual characters (the sexual glands) are concerned. But it is also possible that the determination of the latter—the weighty determination whether the child is to be a boy or a girl—depends on a slight qualitative or quantitative difference in the nuclein or the coloured nuclear matter which comes from both parents in the act of conception.
Figs. 26 and 27.—Impregnation of the ovum of the sea-urchin. (From Hertwig.) In Fig. 26 the little sperm-nucleus (sk) moves towards the larger nucleus of the ovum (ek). In Fig. 27 they nearly touch, and are surrounded by the radiating mantle of protoplasm.
The striking differences of the respective sexual cells in size and shape, which occasioned the erroneous views of earlier scientists, are easily explained on the principle of division of labour. The inert, motionless ovum grows in size according to the quantity of provision it stores up in the form of nutritive yelk for the development of the germ. The active swimming sperm-cell is reduced in size in proportion to its need to seek the ovum and bore its way into its yelk. These differences are very conspicuous in the higher animals, but they are much less in the lower animals. In those protists (unicellular plants and animals) which have the first rudiments of sexual reproduction the two copulating cells are at first quite equal. In these cases the act of impregnation is nothing more than a sudden growth, in which the originally simple cell doubles its volume, and is thus prepared for reproduction (cell-division). Afterwards slight differences are seen in the size of the copulating cells; though the smaller ones still have the same shape as the larger ones. It is only when the difference in size is very pronounced that a notable difference in shape is found: the sprightly sperm-cell changes more in shape and the ovum in size.
Quite in harmony with this new conception of the equivalence of the two gonads, or the equal physiological importance of the male and female sex-cells and their equal share in the process of heredity, is the important fact established by Hertwig (1875), that in normal impregnation only one single spermatozoon copulates with one ovum; the membrane which is raised on the surface of the yelk immediately after one sperm-cell has penetrated (Fig. 25 C) prevents any others from entering. All the rivals of the fortunate penetrator are excluded, and die without. But if the ovum passes into a morbid state, if it is made stiff by a lowering of its temperature or stupefied with narcotics (chloroform, morphia, nicotine, etc.), two or more spermatozoa may penetrate into its yelk-body. We then witness polyspermism. The more Hertwig chloroformed the ovum, the more spermatozoa were able to bore their way into its unconscious body.
Fig. 28—Stem-cell of a rabbit, magnified. In the centre of the granular protoplasm of the fertilised ovum (d) is seen the little, bright stem-nucleus, z is the ovolemma, with a mucous membrane (h). s are dead spermatozoa.
These remarkable facts of impregnation are also of the greatest interest in psychology, especially as regards the theory of the cell-soul, which I consider to be its chief foundation. The phenomena we have described can only be understood and explained by ascribing a certain lower degree of psychic activity to the sexual principles. They feel each other’s proximity, and are drawn together by a sensitive impulse (probably related to smell); they move towards each other, and do not rest until they fuse together. Physiologists may say that it is only a question of a peculiar physico-chemical phenomenon, and not a psychic action; but the two cannot be separated. Even the psychic functions, in the strict sense of the word, are only complex physical processes, or “psycho-physical” phenomena, which are determined in all cases exclusively by the chemical composition of their material substratum.
The monistic view of the matter becomes clear enough when we remember the radical importance of impregnation as regards heredity. It is well known that not only the most delicate bodily structures, but also the subtlest traits of mind, are transmitted from the parents to the children. In this the chromatic matter of the male nucleus is just as important a vehicle as the large caryoplasmic substance of the female nucleus; the one transmits the mental features of the father, and the other those of the mother. The blending of the two parental nuclei determines the individual psychic character of the child.
But there is another important psychological question—the most important of all—that has been definitely answered by the recent discoveries in connection with conception. This is the question of the immortality of the soul. No fact throws more light on it and refutes it more convincingly than the elementary process of conception that we have described. For this copulation of the two sexual nuclei (Figs. 26 and 27) indicates the precise moment at which the individual begins to exist. All the bodily and mental features of the new-born child are the sum-total of the hereditary qualities which it has received in reproduction from parents and ancestors. All that man acquires afterwards in life by the exercise of his organs, the influence of his environment, and education—in a word, by adaptation—cannot obliterate that general outline of his being which he inherited from his parents. But this hereditary disposition, the essence of every human soul, is not “eternal,” but “temporal”; it comes into being only at the moment when the sperm-nucleus of the father and the nucleus of the maternal ovum meet and fuse together. It is clearly irrational to assume an “eternal life without end” for an individual phenomenon, the commencement of which we can indicate to a moment by direct visual observation.
The great importance of the process of impregnation in answering such questions is quite clear. It is true that conception has never been studied microscopically in all its details in the human case—notwithstanding its occurrence at every moment—for reasons that are obvious enough. However, the two cells which need consideration, the female ovum and the male spermatozoon, proceed in the case of man in just the same way as in all the other mammals; the human fœtus or embryo which results from copulation has the same form as with the other animals. Hence, no scientist who is acquainted with the facts doubts that the processes of impregnation are just the same in man as in the other animals.
The stem-cell which is produced, and with which every man begins his career, cannot be distinguished in appearance from those of other mammals, such as the rabbit (Fig. 28). In the case of man, also, this stem-cell differs materially from the original ovum, both in regard to form (morphologically), in regard to material composition (chemically), and in regard to vital properties (physiologically). It comes partly from the father and partly from the mother. Hence it is not surprising that the child who is developed from it inherits from both parents. The vital movements of each of these cells form a sum of mechanical processes which in the last analysis are due to movements of the smallest vital parts, or the molecules, of the living substance. If we agree to call this active substance plasson, and its molecules plastidules, we may say that the individual physiological character of each of these cells is due to its molecular plastidule-movement. Hence, the plastidule-movement of the cytula is the resultant of the combined plastidule-movements of the female ovum and the male sperm-cell.[15]
[15] The plasson of the stem-cell or cytula may, from the anatomical point of view, be regarded as homogeneous and structureless, like that of the monera. This is not inconsistent with our hypothetical ascription to the plastidules (or molecules of the plasson) of a complex molecular structure. The complexity of this is the greater in proportion to the complexity of the organism that is developed from it and the length of the chain of its ancestry, or to the multitude of antecedent processes of heredity and adaptation.
There is a substantial agreement throughout the animal world in the first changes which follow the impregnation of the ovum and the formation of the stem-cell; they begin in all cases with the segmentation of the ovum and the formation of the germinal layers. The only exception is found in the protozoa, the very lowest and simplest forms of animal life; these remain unicellular throughout life. To this group belong the amœbae, gregarinæ, rhizopods, infusoria, etc. As their whole organism consists of a single cell, they can never form germinal layers, or definite strata of cells. But all the other animals—all the tissue-forming animals, or metazoa, as we call them, in contradistinction to the protozoa—construct real germinal layers by the repeated cleavage of the impregnated ovum. This we find in the lower cnidaria and worms, as well as in the more highly-developed molluscs, echinoderms, articulates, and vertebrates.
In all these metazoa, or multicellular animals, the chief embryonic processes are substantially alike, although they often seem to a superficial observer to differ considerably. The stem-cell that proceeds from the impregnated ovum always passes by repeated cleavage into a number of simple cells. These cells are all direct descendants of the stem-cell, and are, for reasons we shall see presently, called segmentation-cells. The repeated cleavage of the stem-cell, which gives rise to these segmentation-spheres, has long been known as “segmentation.” Sooner or later the segmentation-cells join together to form a round (at first, globular) embryonic sphere (blastula); they then form into two very different groups, and arrange themselves in two separate strata—the two primary germinal layers. These enclose a digestive cavity, the primitive gut, with an opening, the primitive mouth. We give the name of the gastrula to the important embryonic form that has these primitive organs, and the name of gastrulation to the formation of it. This ontogenetic process has a very great significance, and is the real starting-point of the construction of the multicellular animal body.
The fundamental embryonic processes of the cleavage of the ovum and the formation of the germinal layers have been very thoroughly studied in the last thirty years, and their real significance has been appreciated. They present a striking variety in the different groups, and it was no light task to prove their essential identity in the whole animal world. But since I formulated the gastræa theory in 1872, and afterwards (1875) reduced all the various forms of segmentation and gastrulation to one fundamental type, their identity may be said to have been established. We have thus mastered the law of unity which governs the first embryonic processes in all the animals.
Man is like all the other higher animals, especially the apes, in regard to these earliest and most important processes. As the human embryo does not essentially differ, even at a much later stage of development—when we already perceive the cerebral vesicles, the eyes, ears, gill-arches, etc.—from the similar forms of the other higher mammals, we may confidently assume that they agree in the earliest embryonic processes, segmentation and the formation of germinal layers. This has not yet, it is true, been established by observation. We have never yet had occasion to dissect a woman immediately after impregnation and examine the stem-cell or the segmentation-cells in her oviduct. However, as the earliest human embryos we have examined, and the later and more developed forms, agree with those of the rabbit, dog, and other higher mammals, no reasonable man will doubt but that the segmentation and formation of layers are the same in both cases.
But the special form of segmentation and layer formation which we find in the mammal is by no means the original, simple, palingenetic form. It has been much modified and cenogenetically altered by a very complex adaptation to embryonic conditions. We cannot, therefore, understand it altogether in itself. In order to do this, we have to make a comparative study of segmentation and layer-formation in the animal world; and we have especially to seek the original, palingenetic form from which the modified cenogenetic (see p. 4) form has gradually been developed.
This original unaltered form of segmentation and layer-formation is found to-day in only one case in the vertebrate-stem to which man belongs—the lowest and oldest member of the stem, the wonderful lancelet or amphioxus (cf. Chapters XVI and XVII). But we find a precisely similar palingenetic form of embryonic development in the case of many of the invertebrate animals, as, for instance, the remarkable ascidia, the pond-snail (Limnæus), and arrow-worm (Sagitta), and many of the echinoderms and cnidaria, such as the common star-fish and sea-urchin, many of the medusæ and corals, and the simpler sponges (Olynthus). We may take as an illustration the palingenetic segmentation and germinal layer-formation in an eight-fold insular coral, which I discovered in the Red Sea, and described as Monoxenia Darwinii.
The impregnated ovum of this coral (Fig. 29 A, B) first splits into two equal cells (C). First, the nucleus of the stem-cell and its central body divide into two halves. These recede from and repel each other, and act as centres of attraction on the surrounding protoplasm; in consequence of this, the protoplasm is constricted by a circular furrow, and, in turn, divides into two halves. Each of the two segmentation-cells thus produced splits in the same way into two equal cells. The four segmentation-cells (grand-daughters of the stem-cell) lie in one plane. Now, however, each of them subdivides into two equal halves, the cleavage of the nucleus again preceding that of the surrounding protoplasm. The eight cells which thus arise break into sixteen, these into thirty-two, and then (each being constantly halved) into sixty-four, 128, and so on.[16] The final result of this repeated cleavage is the formation of a globular cluster of similar segmentation-cells, which we call the mulberry-formation or morula. The cells are thickly pressed together like the parts of a mulberry or blackberry, and this gives a lumpy appearance to the surface of the sphere (Fig. E).[17]
[16] The number of segmentation-cells thus produced increases geometrically in the original gastrulation, or the purest palingenetic form of cleavage. However, in different animals the number reaches a different height, so that the morula, and also the blastula, may consist sometimes of thirty-two, sometimes of sixty-four, and sometimes of 128, or more, cells.
[17] The segmentation-cells which make up the morula after the close of the palingenetic cleavage seem usually to be quite similar, and to present no differences as to size, form, and composition. That, however, does not prevent them from differentiating into animal and vegetative cells, even during the cleavage.
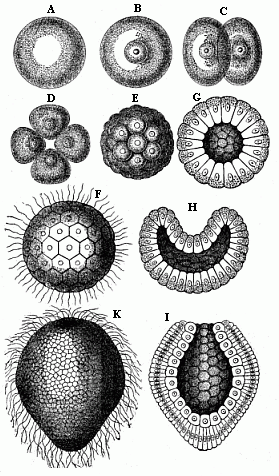
Fig. 29—Gastrulation of a coral (Monoxenia Darwinii). A, B, stem-cell (cytula) or impregnated ovum. In Figure A (immediately after impregnation) the nucleus is invisible. In Figure B (a little later) it is quite clear. C two segmentation-cells. D four segmentation-cells. E mulberry-formation (morula). F blastosphere (blastula). G blastula (transverse section). H depula, or hollowed blastula (transverse section). I gastrula (longitudinal section). K gastrula, or cup-sphere, external appearance.)
When the cleavage is thus ended, the mulberry-like mass changes into a hollow globular sphere. Watery fluid or jelly gathers inside the globule; the segmentation-cells are loosened, and all rise to the surface. There they are flattened by mutual pressure, and assume the shape of truncated pyramids, and arrange themselves side by side in one regular layer (Figs. F, G). This layer of cells is called the germinal membrane (or blastoderm); the homogeneous cells which compose its simple structure are called blastodermic cells; and the whole hollow sphere, the walls of which are made of the preceding, is called the blastula or blastosphere.[18]
[18] The blastula of the lower animals must not be confused with the very different blastula of the mammal, which is properly called the gastrocystis or blastocystis. This cenogenetic gastrocystis and the palingenetic blastula are sometimes very wrongly comprised under the common name of blastula or vesicula blastodermica.
In the case of our coral, and of many other lower forms of animal life, the young embryo begins at once to move independently and swim about in the water. A fine, long, thread-like process, a sort of whip or lash, grows out of each blastodermic cell, and this independently executes vibratory movements, slow at first, but quicker after a time (Fig. F). In this way each blastodermic cell becomes a ciliated cell. The combined force of all these vibrating lashes causes the whole blastula to move about in a rotatory fashion. In many other animals, especially those in which the embryo develops within enclosed membranes, the ciliated cells are only formed at a later stage, or even not formed at all. The blastosphere may grow and expand by the blastodermic cells (at the surface of the sphere) dividing and increasing, and more fluid is secreted in the internal cavity. There are still to-day some organisms that remain throughout life at the structural stage of the blastula—hollow vesicles that swim about by a ciliary movement in the water, the wall of which is composed of a single layer of cells, such as the volvox, the magosphæra, synura, etc. We shall speak further of the great phylogenetic significance of this fact in Chapter XIX.
A very important and remarkable process now follows—namely, the curving or invagination of the blastula (Fig. H). The vesicle with a single layer of cells for wall is converted into a cup with a wall of two layers of cells (cf. Figs. G, H, I). A certain spot at the surface of the sphere is flattened, and then bent inward. This depression sinks deeper and deeper, growing at the cost of the internal cavity. The latter decreases as the hollow deepens. At last the internal cavity disappears altogether, the inner side of the blastoderm (that which lines the depression) coming to lie close on the outer side. At the same time, the cells of the two sections assume different sizes and shapes; the inner cells are more round and the outer more oval (Fig. I). In this way the embryo takes the form of a cup or jar-shaped body, with a wall made up of two layers of cells, the inner cavity of which opens to the outside at one end (the spot where the depression was originally formed). We call this very important and interesting embryonic form the “cup-embryo” or “cup-larva” (gastrula, Fig. 29, I longitudinal section, K external view). I have in my Natural History of Creation given the name of depula to the remarkable intermediate form which appears at the passage of the blastula into the gastrula. In this intermediate stage there are two cavities in the embryo—the original cavity (blastocœl) which is disappearing, and the primitive gut-cavity (progaster) which is forming.
I regard the gastrula as the most important and significant embryonic form in the animal world. In all real animals (that is, excluding the unicellular protists) the segmentation of the ovum produces either a pure, primitive, palingenetic gastrula (Fig. 29 I, K) or an equally instructive cenogenetic form, which has been developed in time from the first, and can be directly reduced to it. It is certainly a fact of the greatest interest and instructiveness that animals of the most different stems—vertebrates and tunicates, molluscs and articulates, echinoderms and annelids, cnidaria and sponges—proceed from one and the same embryonic form. In illustration I give a few pure gastrula forms from various groups of animals (Figs. 30–35, explanation given below each).
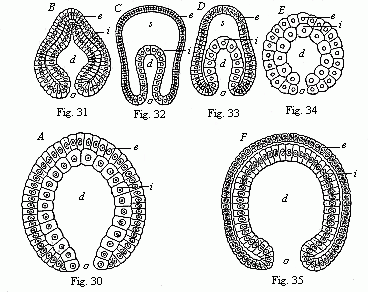
Fig. 30 (A)—Gastrula of a very simple
primitive-gut animal or gastræad (gastrophysema).
(Haeckel.)
Fig. 31 (B)—Gastrula of a worm
(Sagitta). (From Kowalevsky.)
Fig. 32
(C)—Gastrula of an echinoderm (star-fish, Uraster),
not completely folded in (depula). (From Alexander Agassiz.)
Fig.
33 (D)—Gastrula of an arthropod (primitive crab,
Nauplius) (as 32).
Fig. 34 (E)—Gastrula of a
mollusc (pond-snail, Linnæus). (From Karl Rabl.)
Fig. 35
(F)—Gastrula of a vertebrate (lancelet, Amphioxus).
(From Kowalevsky.) (Front view.)
In each figure d is the
primitive-gut cavity, o primitive mouth,
s
segmentation-cavity, i entoderm (gut-layer), e ectoderm (skin
layer).
In view of this extraordinary significance of the gastrula, we must make a very careful study of its original structure. As a rule, the typical gastrula is very small, being invisible to the naked eye, or at the most only visible as a fine point under very favourable conditions, and measuring generally 1/500 to 1/250 of an inch (less frequently 1/50 inch, or even more) in diameter. In shape it is usually like a roundish drinking-cup. Sometimes it is rather oval, at other times more ellipsoid or spindle-shaped; in some cases it is half round, or even almost round, and in others lengthened out, or almost cylindrical.
I give the name of primitive gut (progaster) and primitive mouth (prostoma) to the internal cavity of the gastrula-body and its opening; because this cavity is the first rudiment of the digestive cavity of the organism, and the opening originally served to take food into it. Naturally, the primitive gut and mouth change very considerably afterwards in the various classes of animals. In most of the cnidaria and many of the annelids (worm-like animals) they remain unchanged throughout life. But in most of the higher animals, and so in the vertebrates, only the larger central part of the later alimentary canal develops from the primitive gut; the later mouth is a fresh development, the primitive mouth disappearing or changing into the anus. We must therefore distinguish carefully between the primitive gut and mouth of the gastrula and the later alimentary canal and mouth of the fully developed vertebrate.[19]
[19] My distinction (1872) between the primitive gut and mouth and the later permanent stomach (metagaster) and mouth (metastoma) has been much criticised; but it is as much justified as the distinction between the primitive kidneys and the permanent kidneys. Professor E. Ray-Lankester suggested three years afterwards (1875) the name archenteron for the primitive gut, and blastoporus for the primitive mouth.
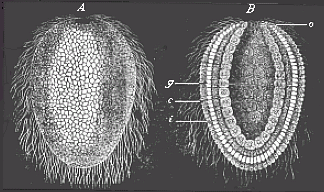
Fig. 36—Gastrula of a lower sponge (lynthus). A external view, B longitudinal section through the axis, g primitive-gut cavity, a primitive mouth-aperture, i inner cell-layer (entoderm, endoblast, gut-layer), e external cell-layer (outer germinal layer, ectoderm, ectoblast, or skin-layer).
The two layers of cells which line the gut-cavity and compose its wall are of extreme importance. These two layers, which are the sole builders of the whole organism, are no other than the two primary germinal layers, or the primitive germ-layers. I have spoken in the introductory section (Chapter III) of their radical importance. The outer stratum is the skin-layer, or ectoderm (Figs. 30–35e); the inner stratum is the gut-layer, or entoderm (i). The former is often also called the ectoblast, or epiblast, and the latter the endoblast, or hypoblast. From these two primary germinal layers alone is developed the entire organism of all the metazoa or multicellular animals. The skin-layer forms the external skin, the gut-layer forms the internal skin or lining of the body. Between these two germinal layers are afterwards developed the middle germinal layer (mesoderma) and the body-cavity (cœloma) filled with blood or lymph.
The two primary germinal layers were first distinguished by Pander in 1817 in the incubated chick. Twenty years later (1849) Huxley pointed out that in many of the lower zoophytes, especially the medusæ, the whole body consists throughout life of these two primary germinal layers. Soon afterwards (1853) Allman introduced the names which have come into general use; he called the outer layer the ectoderm (“outer-skin”), and the inner the entoderm (“inner-skin”). But in 1867 it was shown, particularly by Kowalevsky, from comparative observation, that even in invertebrates, also, of the most different classes—annelids, molluscs, echinoderms, and articulates—the body is developed out of the same two primary layers. Finally, I discovered them (1872) in the lowest tissue-forming animals, the sponges, and proved in my gastræa theory that these two layers must be regarded as identical throughout the animal world, from the sponges and corals to the insects and vertebrates, including man. This fundamental “homology [identity] of the primary germinal layers and the primitive gut” has been confirmed during the last thirty years by the careful research of many able observers, and is now pretty generally admitted for the whole of the metazoa.
As a rule, the cells which compose the two primary germinal layers show appreciable differences even in the gastrula stage. Generally (if not always) the cells of the skin-layer or ectoderm (Figs. 36 c and 37 e) are the smaller, more numerous, and clearer; while the cells of the gut-layer, or entoderm (i), are larger, less numerous, and darker. The protoplasm of the ectodermic (outer) cells is clearer and firmer than the thicker and softer cell-matter of the entodermic (inner) cells; the latter are, as a rule, much richer in yelk-granules (albumen and fatty particles) than the former. Also the cells of the gut-layer have, as a rule, a stronger affinity for colouring matter, and take on a tinge in a solution of carmine, aniline, etc., more quickly and appreciably than the cells of the skin-layer. The nuclei of the entoderm-cells are usually roundish, while those of the ectoderm-cells are oval.
When the doubling-process is complete, very striking histological differences between the cells of the two layers are found (Fig. 37). The tiny, light ectoderm-cells (e) are sharply distinguished from the larger and darker entoderm-cells (i). Frequently this differentiation of the cell-forms sets in at a very early stage, during the segmentation-process, and is already very appreciable in the blastula.
We have, up to the present, only considered that form of segmentation and gastrulation which, for many and weighty reasons, we may regard as the original, primordial, or palingenetic form. We might call it “equal” or homogeneous segmentation, because the divided cells retain a resemblance to each other at first (and often until the formation of the blastoderm). We give the name of the “bell-gastrula,” or archigastrula, to the gastrula that succeeds it. In just the same form as in the coral we considered (Monoxenia, Fig. 29), we find it in the lowest zoophyta (the gastrophysema, Fig. 30), and the simplest sponges (olynthus, Fig. 36); also in many of the medusæ and hydrapolyps, lower types of worms of various classes (brachiopod, arrow-worm, Fig. 31), tunicates (ascidia), many of the echinoderms (Fig. 32), lower articulates (Fig. 33), and molluscs (Fig. 34), and, finally, in a slightly modified form, in the lowest vertebrate (the amphioxus, Fig. 35).

Fig. 37—Cells from the two primary germinal layers of the mammal (from both layers of the blastoderm). i larger and darker cells of the inner stratum, the vegetal layer or entoderm. e smaller and clearer cells from the outer stratum, the animal layer or ectoderm.
The gastrulation of the amphioxus is especially interesting because this lowest and oldest of all the vertebrates is of the highest significance in connection with the evolution of the vertebrate stem, and therefore with that of man (compare Chapters XVI and XVII). Just as the comparative anatomist traces the most elaborate features in the structures of the various classes of vertebrates to divergent development from this simple primitive vertebrate, so comparative embryology traces the various secondary forms of vertebrate gastrulation to the simple, primary formation of the germinal layers in the amphioxus. Although this formation, as distinguished from the cenogenetic modifications of the vertebrate, may on the whole be regarded as palingenetic, it is nevertheless different in some features from the quite primitive gastrulation such as we have, for instance, in the Monoxenia (Fig. 29) and the Sagitta. Hatschek rightly observes that the segmentation of the ovum in the amphioxus is not strictly equal, but almost equal, and approaches the unequal. The difference in size between the two groups of cells continues to be very noticeable in the further course of the segmentation; the smaller animal cells of the upper hemisphere divide more quickly than the larger vegetal cells of the lower (Fig. 38 A, B). Hence the blastoderm, which forms the single-layer wall of the globular blastula at the end of the cleavage-process, does not consist of homogeneous cells of equal size, as in the Sagitta and the Monoxenia; the cells of the upper half of the blastoderm (the mother-cells of the ectoderm) are more numerous and smaller, and the cells of the lower half (the mother-cells of the entoderm) less numerous and larger. Moreover, the segmentation-cavity of the blastula (Fig. 38 C, h) is not quite globular, but forms a flattened spheroid with unequal poles of its vertical axis. While the blastula is being folded into a cup at the vegetal pole of its axis, the difference in the size of the blastodermic cells increases (Fig. 38 D, E); it is most conspicuous when the invagination is complete and the segmentation-cavity has disappeared (Fig. 38 F). The larger vegetal cells of the entoderm are richer in granules, and so darker than the smaller and lighter animal cells of the ectoderm.
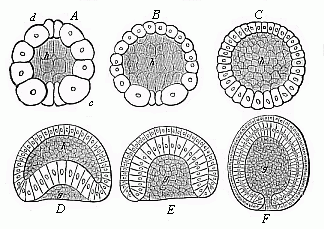
Fig. 38—Gastrulation of the amphioxus, from Hatschek (vertical section through the axis of the ovum). A, B, C three stages in the formation of the blastula; D, E curving of the blastula; F complete gastrula. h segmentation-cavity. g primitive gut-cavity.
But the unequal gastrulation of the amphioxus diverges from the typical equal cleavage of the Sagitta, the Monoxenia (Fig. 29), and the Olynthus (Fig. 36), in another important particular. The pure archigastrula of the latter forms is uni-axial, and it is round in its whole length in transverse section. The vegetal pole of the vertical axis is just in the centre of the primitive mouth. This is not the case in the gastrula of the amphioxus. During the folding of the blastula the ideal axis is already bent on one side, the growth of the blastoderm (or the increase of its cells) being brisker on one side than on the other; the side that grows more quickly, and so is more curved (Fig. 39 v), will be the anterior or belly-side, the opposite, flatter side will form the back (d). The primitive mouth, which at first, in the typical archigastrula, lay at the vegetal pole of the main axis, is forced away to the dorsal side; and whereas its two lips lay at first in a plane at right angles to the chief axis, they are now so far thrust aside that their plane cuts the axis at a sharp angle. The dorsal lip is therefore the upper and more forward, the ventral lip the lower and hinder. In the latter, at the ventral passage of the entoderm into the ectoderm, there lie side by side a pair of very large cells, one to the right and one to the left (Fig. 39 p): these are the important polar cells of the primitive mouth, or “the primitive cells of the mesoderm.” In consequence of these considerable variations arising in the course of the gastrulation, the primitive uni-axial form of the archigastrula in the amphioxus has already become tri-axial, and thus the two-sidedness, or bilateral symmetry, of the vertebrate body has already been determined. This has been transmitted from the amphioxus to all the other modified gastrula-forms of the vertebrate stem.
Apart from this bilateral structure, the gastrula of the amphioxus resembles the typical archigastrula of the lower animals (Figs. 30–36) in developing the two primary germinal layers from a single layer of cells. This is clearly the oldest and original form of the metazoic embryo. Although the animals I have mentioned belong to the most diverse classes, they nevertheless agree with each other, and many more animal forms, in having retained to the present day, by a conservative heredity, this palingenetic form of gastrulation which they have from their earliest common ancestors. But this is not the case with the great majority of the animals. With these the original embryonic process has been gradually more or less altered in the course of millions of years by adaptation to new conditions of development. Both the segmentation of the ovum and the subsequent gastrulation have in this way been considerably changed. In fact, these variations have become so great in the course of time that the segmentation was not rightly understood in most animals, and the gastrula was unrecognised. It was not until I had made an extensive comparative study, lasting a considerable time (in the years 1866–75), in animals of the most diverse classes, that I succeeded in showing the same common typical process in these apparently very different forms of gastrulation, and tracing them all to one original form. I regard all those that diverge from the primary palingenetic gastrulation as secondary, modified, and cenogenetic. The more or less divergent form of gastrula that is produced may be called a secondary, modified gastrula, or a metagastrula. The reader will find a scheme of these different kinds of segmentation and gastrulation at the close of this chapter.
By far the most important process that determines the various cenogenetic forms of gastrulation is the change in the nutrition of the ovum and the accumulation in it of nutritive yelk. By this we understand various chemical substances (chiefly granules of albumin and fat-particles) which serve exclusively as reserve-matter or food for the embryo. As the metazoic embryo in its earlier stages of development is not yet able to obtain its food and so build up the frame, the necessary material has to be stored up in the ovum. Hence we distinguish in the ova two chief elements—the active formative yelk (protoplasm) and the passive food-yelk (deutoplasm, wrongly spoken of as “the yelk”). In the little palingenetic ova, the segmentation of which we have already considered, the yelk-granules are so small and so regularly distributed in the protoplasm of the ovum that the even and repeated cleavage is not affected by them. But in the great majority of the animal ova the food-yelk is more or less considerable, and is stored in a certain part of the ovum, so that even in the unfertilised ovum the “granary” can clearly be distinguished from the formative plasm. As a rule, the formative-yelk (with the germinal vesicle) then usually gathers at one pole and the food-yelk at the other. The first is the animal, and the second the vegetal, pole of the vertical axis of the ovum.
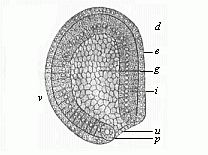
Fig. 39—Gastrula of the amphioxus, seen from left side (diagrammatic median section). (From Hatschek.) g primitive gut, u primitive mouth, p peristomal pole-cells, i entoderm, e ectoderm, d dorsal side, v ventral side.
In these “telolecithal” ova, or ova with the yelk at one end (for instance, in the cyclostoma and amphibia), the gastrulation then usually takes place in such a way that in the cleavage of the impregnated ovum the animal (usually the upper) half splits up more quickly than the vegetal (lower). The contractions of the active protoplasm, which effect this continual cleavage of the cells, meet a greater resistance in the lower vegetal half from the passive deutoplasm than in the upper animal half. Hence we find in the latter more but smaller, and in the former fewer but larger, cells. The animal cells produce the external, and the vegetal cells the internal, germinal layer.
Although this unequal segmentation of the cyclostoma, ganoids, and amphibia seems at first sight to differ from the original equal segmentation (for instance, in the monoxenia, Fig. 29), they both have this in common, that the cleavage process throughout affects the whole cell; hence Remak called it total segmentation, and the ova in question holoblastic, or “whole-cleaving.” It is otherwise with the second chief group of ova, which he distinguished from these as meroblastic, or “partially-cleaving ”: to this class belong the familiar large eggs of birds and reptiles, and of most fishes. The inert mass of the passive food-yelk is so large in these cases that the protoplasmic contractions of the active yelk cannot effect any further cleavage. In consequence, there is only a partial segmentation. While the protoplasm in the animal section of the ovum continues briskly to divide, multiplying the nuclei, the deutoplasm in the vegetal section remains more or less undivided; it is merely consumed as food by the forming cells. The larger the accumulation of food, the more restricted is the process of segmentation. It may, however, continue for some time (even after the gastrulation is more or less complete) in the sense that the vegetal cell-nuclei distributed in the deutoplasm slowly increase by cleavage; as each of them is surrounded by a small quantity of protoplasm, it may afterwards appropriate a portion of the food-yelk, and thus form a real “yelk-cell” (merocyte). When this vegetal cell-formation continues for a long time, after the two primary germinal layers have been formed, it takes the name of the “after-segmentation.”
The meroblastic ova are only found in the larger and more highly developed animals, and only in those whose embryo needs a longer time and richer nourishment within the fœtal membranes. According as the yelk-food accumulates at the centre or at the side of the ovum, we distinguish two groups of dividing ova, periblastic and discoblastic. In the periblastic the food-yelk is in the centre, enclosed inside the ovum (hence they are also called “centrolecithal” ova): the formative yelk surrounds the food-yelk, and so suffers itself a superficial cleavage. This is found among the articulates (crabs, spiders, insects, etc.). In the discoblastic ova the food-yelk gathers at one side, at the vegetal or lower pole of the vertical axis, while the nucleus of the ovum and the great bulk of the formative yelk lie at the upper or animal pole (hence these ova are also called “telolecithal”). In these cases the cleavage of the ovum begins at the upper pole, and leads to the formation of a dorsal discoid embryo. This is the case with all meroblastic vertebrates, most fishes, the reptiles and birds, and the oviparous mammals (the monotremes).
The gastrulation of the discoblastic ova, which chiefly concerns us, offers serious difficulties to microscopic investigation and philosophic consideration. These, however, have been mastered by the comparative embryological research which has been conducted by a number of distinguished observers during the last few decades—especially the brothers Hertwig, Rabl, Kupffer, Selenka, Rückert, Goette, Rauber, etc. These thorough and careful studies, aided by the most perfect modern improvements in technical method (in tinting and dissection), have given a very welcome support to the views which I put forward in my work, On the Gastrula and the Segmentation of the Animal Ovum [not translated], in 1875. As it is very important to understand these views and their phylogenetic foundation clearly, not only as regards evolution in general, but particularly in connection with the genesis of man, I will give here a brief statement of them as far as they concern the vertebrate-stem:—
1. All the vertebrates, including man, are phylogenetically (or genealogically) related—that is, are members of one single natural stem.
2. Consequently, the embryonic features in their individual development must also have a genetic connection.
3. As the gastrulation of the amphioxus shows the original palingenetic form in its simplest features, that of the other vertebrates must have been derived from it.
4. The cenogenetic modifications of the latter are more appreciable the more food-yelk is stored up in the ovum.
5. Although the mass of the food-yelk may be very large in the ova of the discoblastic vertebrates, nevertheless in every case a blastula is developed from the morula, as in the holoblastic ova.
6. Also, in every case, the gastrula develops from the blastula by curving or invagination.
7. The cavity which is produced in the fœtus by this curving is, in each case, the primitive gut (progaster), and its opening the primitive mouth (prostoma).
8. The food-yelk, whether large or small, is always stored in the ventral wall of the primitive gut; the cells (called “merocytes”) which may be formed in it subsequently (by “after-segmentation”) also belong to the inner germinal layer, like the cells which immediately enclose the primitive gut-cavity.
9. The primitive mouth, which at first lies below at the lower pole of the vertical axis, is forced, by the growth of the yelk, backwards and then upwards, towards the dorsal side of the embryo; the vertical axis of the primitive gut is thus gradually converted into horizontal.
10. The primitive mouth is closed sooner or later in all the vertebrates, and does not evolve into the permanent mouth-aperture; it rather corresponds to the “properistoma,” or region of the anus. From this important point the formation of the middle germinal layer proceeds, between the two primary layers.
The wide comparative studies of the scientists I have named have further shown that in the case of the discoblastic higher vertebrates (the three classes of amniotes) the primitive mouth of the embryonic disc, which was long looked for in vain, is found always, and is nothing else than the familiar “primitive groove.” Of this we shall see more as we proceed. Meantime we realise that gastrulation may be reduced to one and the same process in all the vertebrates. Moreover, the various forms it takes in the invertebrates can always be reduced to one of the four types of segmentation described above. In relation to the distinction between total and partial segmentation, the grouping of the various forms is as follows:—
| I. Palingenetic (primitive) segmentation. | 1.
Equal segmentation (bell-gastrula). | A. Total segmentation (without independent food-yelk). |
| II. Cenogenetic segmentation (modified by adaptation). | 2. Unequal segmentation (hooded gastrula). | |
| 3. Discoid segmentation (discoid gastrula). | B. Partial
segmentation (with independent food-yelk). | |
| 4. Superficial segmentation (spherical gastrula). |
The lowest metazoa we know—namely, the lower zoophyta (sponges, simple polyps, etc.)—remain throughout life at a stage of development which differs little from the gastrula; their whole body consists of two layers of cells. This is a fact of extreme importance. We see that man, and also other vertebrates, pass quickly through a stage of development in which they consist of two layers, just as these lower zoophyta do throughout life. If we apply our biogenetic law to the matter, we at once reach this important conclusion. “Man and all the other animals which pass through the two-layer stage, or gastrula-form, in the course of their embryonic development, must descend from a primitive simple stem-form, the whole body of which consisted throughout life (as is the case with the lower zoophyta to-day) merely of two cell-strata or germinal layers.” We will call this primitive stem-form, with which we shall deal more fully later on, the gastræa—that is to say, “primitive-gut animal.”
According to this gastræa-theory there was originally in all the multicellular animals one organ with the same structure and function. This was the primitive gut; and the two primary germinal layers which form its wall must also be regarded as identical in all. This important homology or identity of the primary germinal layers is proved, on the one hand, from the fact that the gastrula was originally formed in the same way in all cases—namely, by the curving of the blastula; and, on the other hand, by the fact that in every case the same fundamental organs arise from the germinal layers. The outer or animal layer, or ectoderm, always forms the chief organs of animal life—the skin, nervous system, sense-organs, etc.; the inner or vegetal layer, or entoderm, gives rise to the chief organs of vegetative life—the organs of nourishment, digestion, blood-formation, etc.
In the lower zoophyta, whose body remains at the two-layer stage throughout life, the gastræads, the simplest sponges (Olynthus), and polyps (Hydra), these two groups of functions, animal and vegetative, are strictly divided between the two simple primary layers. Throughout life the outer or animal layer acts simply as a covering for the body, and accomplishes its movement and sensation. The inner or vegetative layer of cells acts throughout life as a gut-lining, or nutritive layer of enteric cells, and often also yields the reproductive cells.
The best known of these “gastræads,” or “gastrula-like animals,” is the common fresh-water polyp (Hydra). This simplest of all the cnidaria has, it is true, a crown of tentacles round its mouth. Also its outer germinal layer has certain special modifications. But these are secondary additions, and the inner germinal layer is a simple stratum of cells. On the whole, the hydra has preserved to our day by heredity the simple structure of our primitive ancestor, the gastræa (cf. Chapter XIX).
In all other animals, particularly the vertebrates, the gastrula is merely a brief transitional stage. Here the two-layer stage of the embryonic development is quickly succeeded by a three-layer, and then a four-layer, stage. With the appearance of the four superimposed germinal layers we reach again a firm and steady standing-ground, from which we may follow the further, and much more difficult and complicated, course of embryonic development.
SUMMARY OF THE CHIEF DIFFERENCES IN THE OVUM-SEGMENTATION AND GASTRULATION OF
ANIMALS.
The animal stems are indicated by the
letters a–g: a Zoophyta. b Annelida.
c
Mollusca. d Echinoderma. e Articulata. f Tunicata.
g Vertebrata.
| I. Total Segmentation. Holoblastic ova. Gastrula without separate food-yelk. Hologastrula. | I. Primitive Segmentation. Archiblastic ova. Bell-gastrula (archigastrula.) | a. Many lower zoophyta
(sponges, hydrapolyps, medusæ, simpler corals). b. Many lower annelids (sagitta, phoronis, many nematoda, etc., terebratula, argiope, pisidium). c. Some lower molluscs. d. Many echinoderms. e. A few lower articulata (some brachiopods, copepods: Tardigrades, pteromalina). f. Many tunicata. g. The acrania (amphioxus). |
| II. Unequal Segmentation. Amphiblastic ova. Hooded-gastrula (amphigastrula). |
a. Many zoophyta (sponges, medusæ, corals, siphonophoræ, ctenophora). b. Most worms. c. Most molluscs. d. Many echinoderms (viviparous species and some others). e. Some of the lower articulata (both crustacea and tracheata). f. Many tunicata. g. Cyclostoma, the oldest fishes, amphibia, mammals (not including man). | |
| II. Partial Segmentation. Meroblastic ova. Gastrula with separate food-yelk. Merogastrula. | III. Discoid Segmentation. Discoblastic ova. Discoid gastrula. | c.
Cephalopods or cuttlefish. e. Many articulata, wood-lice, scorpions, etc. g. Primitive fishes, bony fishes, reptiles, birds, monotremes. |
| IV. Superficial Segmentation. Periblastic ova. Spherical-gastrula. |
e. The great majority of the articulata (crustaceans, myriapods, arachnids, insects). |
[20] Cf. Balfour’s Manual of Comparative Embryology, vol. ii; Theodore Morgan’s The Development of the Frog’s Egg.
The remarkable processes of gastrulation, ovum-segmentation, and formation of germinal layers present a most conspicuous variety. There is to-day only the lowest of the vertebrates, the amphioxus, that exhibits the original form of those processes, or the palingenetic gastrulation which we have considered in the preceding chapter, and which culminates in the formation of the archigastrula (Fig. 38). In all other extant vertebrates these fundamental processes have been more or less modified by adaptation to the conditions of embryonic development (especially by changes in the food-yelk); they exhibit various cenogenetic types of the formation of germinal layers. However, the different classes vary considerably from each other. In order to grasp the unity that underlies the manifold differences in these phenomena and their historical connection, it is necessary to bear in mind always the unity of the vertebrate-stem. This “phylogenetic unity,” which I developed in my General Morphology in 1866, is now generally admitted. All impartial zoologists agree to-day that all the vertebrates, from the amphioxus and the fishes to the ape and man, descend from a common ancestor, “the primitive vertebrate.” Hence the embryonic processes, by which each individual vertebrate is developed, must also be capable of being reduced to one common type of embryonic development; and this primitive type is most certainly exhibited to-day by the amphioxus.
It must, therefore, be our next task to make a comparative study of the various forms of vertebrate gastrulation, and trace them backwards to that of the lancelet. Broadly speaking, they fall first into two groups: the older cyclostoma, the earliest fishes, most of the amphibia, and the viviparous mammals, have holoblastic ova—that is to say, ova with total, unequal segmentation; while the younger cyclostoma, most of the fishes, the cephalopods, reptiles, birds, and monotremes, have meroblastic ova, or ova with partial discoid segmentation. A closer study of them shows, however, that these two groups do not present a natural unity, and that the historical relations between their several divisions are very complicated. In order to understand them properly, we must first consider the various modifications of gastrulation in these classes. We may begin with that of the amphibia.
The most suitable and most available objects of study in this class are the eggs of our indigenous amphibia, the tailless frogs and toads, and the tailed salamander. In spring they are to be found in clusters in every pond, and careful examination of the ova with a lens is sufficient to show at least the external features of the segmentation. In order to understand the whole process rightly and follow the formation of the germinal layers and the gastrula, the ova of the frog and salamander must be carefully hardened; then the thinnest possible sections must be made of the hardened ova with the microtome, and the tinted sections must be very closely compared under a powerful microscope.
The ova of the frog or toad are globular in shape, about the twelfth of an inch in diameter, and are clustered in jelly-like masses, which are lumped together in the case of the frog, but form long strings in the case of the toad. When we examine the opaque, grey, brown, or blackish ova closely, we find that the upper half is darker than the lower. The middle of the upper half is in many species black, while the middle of the lower half is white.[21] In this way we get a definite axis of the ovum with two poles. To give a clear idea of the segmentation of this ovum, it is best to compare it with a globe, on the surface of which are marked the various parallels of longitude and latitude. The superficial dividing lines between the different cells, which come from the repeated segmentation of the ovum, look like deep furrows on the surface, and hence the whole process has been given the name of furcation. In reality, however, this “furcation,” which was formerly regarded as a very mysterious process, is nothing but the familiar, repeated cell-segmentation. Hence also the segmentation-cells which result from it are real cells.
[21] The colouring of the eggs of the amphibia is caused by the accumulation of dark-colouring matter at the animal pole of the ovum. In consequence of this, the animal cells of the ectoderm are darker than the vegetal cells of the entoderm. We find the reverse of this in the case of most animals, the protoplasm of the entoderm cells being usually darker and coarser-grained.
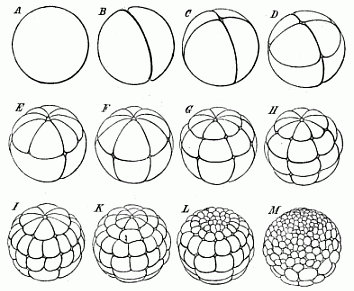
Fig. 40—The cleavage of the frog’s ovum (magnified). A stem-cell. B the first two segmentation-cells. C four cells. D eight cells (4 animal and 4 vegetative). E twelve cells (8 animal and 4 vegetative). F sixteen cells (8 animal and 8 vegetative). G twenty-four cells (16 animal and 8 vegetative). H thirty-two cells. I forty-eight cells. K sixty-four cells. L ninety-six cells. M 160 cells (128 animal and 32 vegetative).
The unequal segmentation which we observe in the ovum of the amphibia has the special feature of beginning at the upper and darker pole (the north pole of the terrestrial globe in our illustration), and slowly advancing towards the lower and brighter pole (the south pole). Also the upper and darker hemisphere remains in this position throughout the course of the segmentation, and its cells multiply much more briskly. Hence the cells of the lower hemisphere are found to be larger and less numerous. The cleavage of the stem-cell (Fig. 40 A) begins with the formation of a complete furrow, which starts from the north pole and reaches to the south (B). An hour later a second furrow arises in the same way, and this cuts the first at a right angle (Fig. 40 C). The ovum is thus divided into four equal parts. Each of these four “segmentation cells” has an upper and darker and a lower, brighter half. A few hours later a third furrow appears, vertically to the first two (Fig. 40 D). The globular germ now consists of eight cells, four smaller ones above (northern) and four larger ones below (southern). Next, each of the four upper ones divides into two halves by a cleavage beginning from the north pole, so that we now have eight above and four below (Fig. 40 E). Later, the four new longitudinal divisions extend gradually to the lower cells, and the number rises from twelve to sixteen (F). Then a second circular furrow appears, parallel to the first, and nearer to the north pole, so that we may compare it to the north polar circle. In this way we get twenty-four segmentation-cells—sixteen upper, smaller, and darker ones, and eight smaller and brighter ones below (G). Soon, however, the latter also sub-divide into sixteen, a third or “meridian of latitude” appearing, this time in the southern hemisphere: this makes thirty-two cells altogether (H). Then eight new longitudinal lines are formed at the north pole, and these proceed to divide, first the darker cells above and afterwards the lighter southern cells, and finally reach the south pole. In this way we get in succession forty, forty-eight, fifty-six, and at last sixty-four cells (I, K). In the meantime, the two hemispheres differ more and more from each other. Whereas the sluggish lower hemisphere long remains at thirty-two cells, the lively northern hemisphere briskly sub-divides twice, producing first sixty-four and then 128 cells (L, M). Thus we reach a stage in which we count on the surface of the ovum 128 small cells in the upper half and thirty-two large ones in the lower half, or 160 altogether. The dissimilarity of the two halves increases: while the northern breaks up into a great number of small cells, the southern consists of a much smaller number of larger cells. Finally, the dark cells of the upper half grow almost over the surface of the ovum, leaving only a small circular spot
at the south pole, where the large and clear cells of the lower half are visible. This white region at the south pole corresponds, as we shall see afterwards, to the primitive mouth of the gastrula. The whole mass of the inner and larger and clearer cells (including the white polar region) belongs to the entoderm or ventral layer. The outer envelope of dark smaller cells forms the ectoderm or skin-layer.
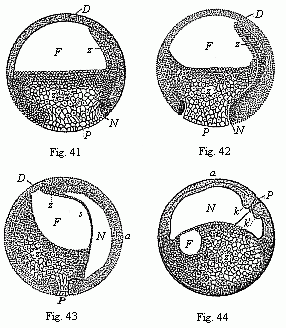
Figs. 41–44—Four vertical sections of the fertilised ovum of the toad, in four successive stages of development. The letters have the same meaning throughout: F segmentation-cavity. D covering of same (D dorsal half of the embryo, P ventral half). P yelk-stopper (white round field at the lower pole). Z yelk-cells of the entoderm (Remak’s “glandular embryo”). N primitive gut cavity (progaster or Rusconian alimentary cavity). The primitive mouth (prostoma) is closed by the yelk-stopper, P. s partition between the primitive gut cavity (N) and the segmentation cavity (F). k k′, section of the large circular lip-border of the primitive mouth (the Rusconian anus). The line of dots between k and k′ indicates the earlier connection of the yelk-stopper (P) with the central mass of the yelk-cells (Z). In Fig. 44 the ovum has turned 90°, so that the back of the embryo is uppermost and the ventral side down. (From Stricker.).
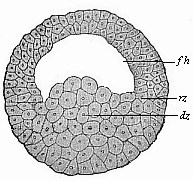
Fig. 45—Blastula of the water-salamander (Triton). fh segmentation-cavity, dz yelk-cells, rz border-zone. (From Hertwig.)
In the meantime, a large cavity, full of fluid, has been formed within the globular body—the segmentation-cavity or embryonic cavity (blastocœl, Figs. 41–44 F). It extends considerably as the cleavage proceeds, and afterwards assumes an almost semi-circular form (Fig. 41 F). The frog-embryo now represents a modified embryonic vesicle or blastula, with hollow animal half and solid vegetal half.
Now a second, narrower but longer, cavity arises by a process of folding at the lower pole, and by the falling away from each other of the white entoderm-cells (Figs. 41–44 N). This is the primitive gut-cavity or the gastric cavity of the gastrula, progaster or archenteron. It was first observed in the ovum of the amphibia by Rusconi, and so called the Rusconian cavity. The reason of its peculiar narrowness here is that it is, for the most part, full of yelk-cells of the entoderm. These also stop up the whole of the wide opening of the primitive mouth, and form what is known as the “yelk-stopper,” which is seen freely at the white round spot at the south pole (P). Around it the ectoderm is much thicker, and forms the border of the primitive mouth, the most important part of the embryo (Fig. 44 k, k′). Soon the primitive gut-cavity stretches further and further at the expense of the segmentation-cavity (F), until at last the latter disappears altogether. The two cavities are only separated by a thin partition (Fig. 43 s). With the formation of the primitive gut our frog-embryo has reached the gastrula stage, though it is clear that this cenogenetic amphibian gastrula is very different from the real palingenetic gastrula we have considered (Figs. 30–36).
In the growth of this hooded gastrula we cannot sharply mark off the various stages which we distinguish successively in the bell-gastrula as morula and gastrula. Nevertheless, it is not difficult to reduce the whole cenogenetic or disturbed development of this amphigastrula to the true palingenetic formation of the archigastrula of the amphioxus.
Fig. 46—Embryonic vesicle of triton (blastula), outer view, with the transverse fold of the primitive mouth (u). (From Hertwig.)
This reduction becomes easier if, after considering the gastrulation of the tailless amphibia (frogs and toads), we glance for a moment at that of the tailed amphibia, the salamanders. In some of the latter, that have only recently been carefully studied, and that are phylogenetically older, the process is much simpler and clearer than is the case with the former and longer known. Our common water-salamander (Triton taeniatus) is a particularly good subject for observation. Its nutritive yelk is much smaller and its formative yelk less obscured with black pigment-cells than in the case of the frog; and its gastrulation has better retained the original palingenetic character. It was first described by Scott and Osborn (1879), and Oscar Hertwig especially made a careful study of it (1881), and rightly pointed out its great importance in helping us to understand the vertebrate development. Its globular blastula (Fig. 45) consists of loosely-aggregated, yelk-filled entodermic cells or yelk-cells (dz) in the lower vegetal half; the upper, animal half encloses the hemispherical segmentation-cavity (fh), the curved roof of which is formed of two or three strata of small ectodermic cells. At the point where the latter pass into the former (at the equator of the globular vesicle) we have the border zone (rz). The folding which leads to the formation of the gastrula takes place at a spot in this border zone, the primitive mouth (Fig. 46 u).
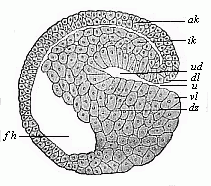
Fig. 47—Sagittal section of a hooded-embryo (depula) of triton (blastula at the commencement of gastrulation). ak outer germinal layer, ik inner germinal layer, fh segmentation-cavity, ud primitive gut, u primitive mouth, dl and vl dorsal and ventral lips of the mouth, dz yelk-cells. (From Hertwig.)
Unequal segmentation takes place in some of the cyclostoma and in the oldest fishes in just the same way as in most of the amphibia. Among the cyclostoma (“round-mouthed”) the familiar lampreys are particularly interesting. In respect of organisation and development they are half-way between the acrania (lancelet) and the lowest real fishes (Selachii); hence I divided the group of the cyclostoma in 1886 from the real fishes with which they were formerly associated, and formed of them a special class of vertebrates. The ovum-segmentation in our common river-lamprey (Petromyzon fluviatilis) was described by Max Schultze in 1856, and afterwards by Scott (1882) and Goette (1890).
Unequal total segmentation follows the same lines in the oldest fishes, the selachii and ganoids, which are directly descended from the cyclostoma. The primitive fishes (Selachii), which we must regard as the ancestral group of the true fishes, were generally considered, until a short time ago, to be discoblastic. It was not until the beginning of the twentieth century that Bashford Dean made the important discovery in Japan that one of the oldest living fishes of the shark type (Cestracion japonicus) has the same total unequal segmentation as the amphiblastic plated fishes (ganoides).[22] This is particularly interesting in connection with our subject, because the few remaining survivors of this division, which was so numerous in paleozoic times, exhibit three different types of gastrulation.
[22] Bashford Dean, Holoblastic Cleavage in the Egg of a Shark, Cestracion japonicus Macleay. Annotationes zoologicae japonenses, vol. iv, Tokio, 1901.
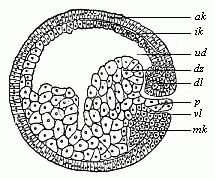
Fig. 48—Sagittal section of the gastrula of the water-salamander (Triton). (From Hertwig.) Letters as in Fig. 47; except—p yelk-stopper, mk beginning of the middle germinal layer.)
The oldest and most conservative forms of the modern ganoids are the scaly sturgeons (Sturiones), plated fishes of great evolutionary importance, the eggs of which are eaten as caviar; their cleavage is not essentially different from that of the lampreys and the amphibia. On the other hand, the most modern of the plated fishes, the beautifully scaled bony pike of the North American rivers (Lepidosteus), approaches the osseous fishes, and is discoblastic like them. A third genus (Amia) is midway between the sturgeons and the latter.
The group of the lung-fishes (Dipneusta or Dipnoi) is closely connected with the older ganoids. In respect of their whole organisation they are midway between the gill-breathing fishes and the lung-breathing amphibia; they share with the former the shape of the body and limbs, and with the latter the form of the heart and lungs. Of the older dipnoi (Paladipneusta) we have now only one specimen, the remarkable Ceratodus of East Australia; its amphiblastic gastrulation has been recently explained by Richard Semon (cf. Chapter XXI). That of the two modern dipneusta, of which Protopterus is found in Africa and Lepidosiren in America, is not materially different. (Cf. Fig. 51.)
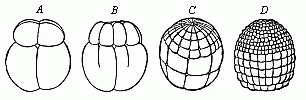
Fig. 49—Ovum-segmentation of the lamprey (Petromyzon fluviatalis), in four successive stages. The small cells of the upper (animal) hemisphere divide much more quickly than the cells of the lower (vegetal) hemisphere.
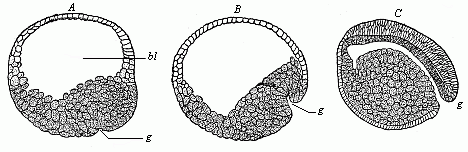
Fig. 50—Gastrulation of the lamprey (Petromyzon fluviatilis). A blastula, with wide embryonic cavity (blastocoel, bl), g incipient invagination. B depula, with advanced invagination, from the primitive mouth (g). C gastrula, with complete primitive gut: the embryonic cavity has almost disappeared in consequence of invagination.
All these amphiblastic vertebrates, Petromyzon and Cestracion, Accipenser and Ceratodus, and also the salamanders and batrachia, belong to the old, conservative groups of our stem. Their unequal ovum-segmentation and gastrulation have many peculiarities in detail, but can always be reduced with comparative ease to the original cleavage and gastrulation of the lowest vertebrate, the amphioxus; and this is little removed, as we have seen, from the very simple archigastrula of the Sagitta and Monoxenia (see Fig. 29–36). All these and many other classes of animals generally agree in the circumstance that in segmentation their ovum divides into a large number of cells by repeated cleavage. All such ova have been called, after Remak, “whole-cleaving” (holoblasta), because their division into cells is complete or total.
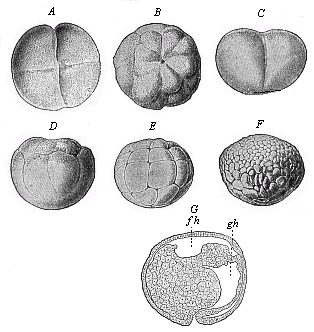
Fig. 51—Gastrulation of ceratodus (from Semon). A and C stage with four cells, B and D with sixteen cells. A and B are seen from above, C and D sideways. E stage with thirty-two cells; F blastula; G gastrula in longitudinal section. fh segmentation-cavity. gh primitive gut or gastric cavity.
In a great many other classes of animals this is not the case, as we find (in the vertebrate stem) among the birds, reptiles, and most of the fishes; among the insects and most of the spiders and crabs (of the articulates); and the cephalopods (of the molluscs). In all these animals the mature ovum, and the stem-cell that arises from it in fertilisation, consist of two different and separate parts, which we have called formative yelk and nutritive yelk. The formative yelk alone consists of living protoplasm, and is the active, evolutionary, and nucleated part of the ovum; this alone divides in segmentation, and produces the numerous cells which make up the embryo. On the other hand, the nutritive yelk is merely a passive part of the contents of the ovum, a subordinate element which contains nutritive material (albumin, fat, etc.), and so represents in a sense the provision-store of the developing embryo. The latter takes a quantity of food out of this store, and finally consumes it all. Hence the nutritive yelk is of great indirect importance in embryonic development, though it has no direct share in it. It either does not divide at all, or only later on, and does not generally consist of cells. It is sometimes large and sometimes small, but generally many times larger than the formative yelk; and hence it is that it was formerly thought the more important of the two. As the respective significance of these two parts of the ovum is often wrongly described, it must be borne in mind that the nutritive yelk is only a secondary addition to the primary cell, it is an inner enclosure, not an external appendage. All ova that have this independent nutritive yelk are called, after Remak, “partially-cleaving” (meroblasta). Their segmentation is incomplete or partial.
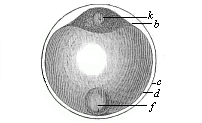
Fig. 52—Ovum of a deep-sea bony fish. b protoplasm of the stem-cell, k nucleus of same, d clear globule of albumin, the nutritive yelk, f fat-globule of same, c outer membrane of the ovum, or ovolemma.)
There are many difficulties in the way of understanding this partial segmentation and the gastrula that arises from it. We have only recently succeeded, by means of comparative research, in overcoming these difficulties, and reducing this cenogenetic form of gastrulation to the original palingenetic type. This is comparatively easy in the small meroblastic ova which contain little nutritive yelk—for instance, in the marine ova of a bony fish, the development of which I observed in 1875 at Ajaccio in Corsica. I found them joined together in lumps of jelly, floating on the surface of the sea; and, as the little ovula were completely transparent, I could easily follow the development of the germ step by step. These ovula are glossy and colourless globules of little more than the 50th of an inch. Inside a structureless, thin, but firm membrane (ovolemma, Fig. 52 c) we find a large, quite clear, and transparent globule of albumin (d). At both poles of its axis this globule has a pit-like depression. In the pit at the upper, animal pole (which is turned downwards in the floating ovum) there is a bi-convex lens composed of protoplasm, and this encloses the nucleus (k); this is the formative yelk of the stem-cell, or the germinal disk (b). The small fat-globule (f) and the large albumin-globule (d) together form the nutritive yelk. Only the formative yelk undergoes cleavage, the nutritive yelk not dividing at all at first.
The segmentation of the lens-shaped formative yelk (b) proceeds quite independently of the nutritive yelk, and in perfect geometrical order.
When the mulberry-like cluster of cells has been formed, the border-cells of the lens separate from the rest and travel into the yelk and the border-layer. From this the blastula is developed; the regular bi-convex lens being converted into a disk, like a watch-glass, with thick borders. This lies on the upper and less curved polar surface of the nutritive yelk like the watch glass on the yelk. Fluid gathers between the outer layer and the border, and the segmentation-cavity is formed. The gastrula is then formed by invagination, or a kind of turning-up of the edge of the blastoderm. In this process the segmentation-cavity disappears.
The space underneath the entoderm corresponds to the primitive gut-cavity, and is filled with the decreasing food-yelk (n). Thus the formation of the gastrula of our fish is complete. In contrast to the two chief forms of gastrula we considered previously, we give the name of discoid gastrula (discogastrula, Fig. 54) to this third principal type.
Very similar to the discoid gastrulation of the bony fishes is that of the hags or myxinoida, the remarkable cyclostomes that live parasitically in the body-cavity of fishes, and are distinguished by several notable peculiarities from their nearest relatives, the lampreys. While the amphiblastic ova of the latter are small and develop like those of the amphibia, the cucumber-shaped ova of the hag are about an inch long, and form a discoid gastrula. Up to the present it has only been observed in one species (Bdellostoma Stouti), by Dean and Doflein (1898).
It is clear that the important features which distinguish the discoid gastrula from the other chief forms we have considered are determined by the large food-yelk. This takes no direct part in the building of the germinal layers, and completely fills the primitive gut-cavity of the gastrula, even protruding at the mouth-opening. If we imagine the original bell-gastrula (Figs. 30–36) trying to swallow a ball of food which is much bigger than itself, it would spread out round it in discoid shape in the attempt, just as we find to be the case here (Fig. 54). Hence we may derive the discoid gastrula from the original bell-gastrula, through the intermediate stage of the hooded gastrula. It has arisen through the accumulation of a store of food-stuff at the vegetal pole, a “nutritive yelk” being thus formed in contrast to the “formative yelk.” Nevertheless, the gastrula is formed here, as in the previous cases, by the folding or invagination of the blastula. We can, therefore, reduce this cenogenetic form of the discoid segmentation to the palingenetic form of the primitive cleavage.
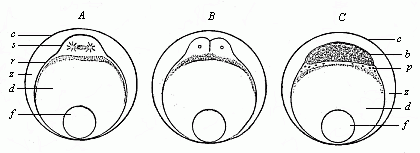
Fig. 53—Ovum-segmentation of a bony fish. A first cleavage of the stem-cell (cytula), B division of same into four segmentation-cells (only two visible), C the germinal disk divides into the blastoderm (b) and the periblast (p). d nutritive yelk, f fat-globule, c ovolemma, z space between the ovolemma and the ovum, filled with a clear fluid.)
This reduction is tolerably easy and confident in the case of the small ovum of our deep-sea bony fish, but it becomes difficult and uncertain in the case of the large ova that we find in the majority of the other fishes and in all the reptiles and birds. In these cases the food-yelk is, in the first place, comparatively colossal, the formative yelk being almost invisible beside it; and, in the second place, the food-yelk contains a quantity of different elements, which are known as “yelk-granules, yelk-globules, yelk-plates, yelk-flakes, yelk-vesicles,” and so on. Frequently these definite elements in the yelk have been described as real cells, and it has been wrongly stated that a portion of the embryonic body is built up from these cells. This is by no means the case. In every case, however large it is—and even when cell-nuclei travel into it during the cleavage of the border—the nutritive yelk remains a dead accumulation of food, which is taken into the gut during embryonic development and consumed by the embryo. The latter develops solely from the living formative yelk of the stem-cell. This is equally true of the ova of our small bony fishes and of the colossal ova of the primitive fishes, reptiles, and birds.
The gastrulation of the primitive fishes or selachii (sharks and rays) has been carefully studied of late years by Ruckert, Rabl, and H.E. Ziegler in particular, and is very important in the sense that this group is the oldest among living fishes, and their gastrulation can be derived directly from that of the cyclostoma by the accumulation of a large quantity of food-yelk. The oldest sharks (Cestracion) still have the unequal segmentation inherited from the cyclostoma. But while in this case, as in the case of the amphibia, the small ovum completely divides into cells in segmentation, this is no longer so in the great majority of the selachii (or Elasmobranchii). In these the contractility of the active protoplasm no longer suffices to break up the huge mass of the passive deutoplasm completely into cells; this is only possible in the upper or dorsal part, but not in the lower or ventral section. Hence we find in the primitive fishes a blastula with a small eccentric segmentation-cavity (Fig. 55 b), the wall of which varies greatly in composition. The circular border of the germinal disk which connects the roof and floor of the segmentation-cavity corresponds to the border-zone at the equator of the amphibian ovum. In the middle of its hinder border we have the beginning of the invagination of the primitive gut (Fig. 56 ud); it extends gradually from this spot (which corresponds to the Rusconian anus of the amphibia) forward and around, so that the primitive mouth becomes first crescent-shaped and then circular, and, as it opens wider, surrounds the ball of the larger food-yelk.
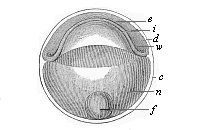
Fig. 54—Discoid gastrula (discogastrula) of a bony fish. e ectoderm, i entoderm, w border-swelling or primitive mouth, n albuminous globule of the nutritive yelk, f fat-globule of same, c external membrane (ovolemma), d partition between entoderm and ectoderm (earlier the segmentation-cavity.)
Essentially different from the wide-mouthed discoid gastrula of most of the selachii is the narrow-mouthed discoid gastrula (or epigastrula) of the amniotes, the reptiles, birds, and monotremes; between the two—as an intermediate stage—we have the amphigastrula of the amphibia. The latter has developed from the amphigastrula of the ganoids and dipneusts, whereas the discoid amniote gastrula has been evolved from the amphibian gastrula by the addition of food-yelk. This change of gastrulation is still found in the remarkable ophidia (Gymnophiona, Cœcilia, or Peromela), serpent-like amphibia that live in moist soil in the tropics, and in many respects represent the transition from the gill-breathing amphibia to the lung-breathing reptiles. Their embryonic development has been explained by the fine studies of the brothers Sarasin of Ichthyophis glutinosa at Ceylon (1887), and those of August Brauer of the Hypogeophis rostrata in the Seychelles (1897). It is only by the historical and comparative study of these that we can understand the difficult and obscure gastrulation of the amniotes.
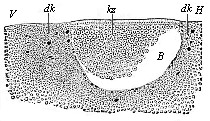
Fig. 55—Longitudinal section through the blastula of a shark (Pristiuris). (From Ruckert.) (Looked at from the left; to the right is the hinder end, H, to the left the fore end, V.) B segmentation-cavity, kz cells of the germinal membrane, dk yelk-nuclei.
The bird’s egg is particularly important for our purpose, because most of the chief studies of the development of the vertebrates are based on observations of the hen’s egg during hatching. The mammal ovum is much more difficult to obtain and study, and for this practical and obvious reason very rarely thoroughly investigated. But we can get hens’ eggs in any quantity at any time, and, by means of artificial incubation, follow the development of the embryo step by step. The bird’s egg differs considerably from the tiny mammal ovum in size, a large quantity of food-yelk accumulating within the original yelk or the protoplasm of the ovum. This is the yellow ball which we commonly call the yolk of the egg. In order to understand the bird’s egg aright—for it is very often quite wrongly explained—we must examine it in its original condition, and follow it from the very beginning of its development in the bird’s ovary. We then see that the original ovum is a quite small, naked, and simple cell with a nucleus, not differing in either size or shape from the original ovum of the mammals and other animals (cf. Fig. 13 E). As in the case of all the craniota (animals with a skull), the original or primitive ovum (protovum) is covered with a continuous layer of small cells. This membrane is the follicle, from which the ovum afterwards issues. Immediately underneath it the structureless yelk-membrane is secreted from the yelk.
The small primitive ovum of the bird begins very early to take up into itself a quantity of food-stuff through the yelk-membrane, and work it up into the “yellow yelk.” In this way the ovum enters on its second stage (the metovum), which is many times larger than the first, but still only a single enlarged cell. Through the accumulation of the store of yellow yelk within the ball of protoplasm the nucleus it contains (the germinal vesicle) is forced to the surface of the ball. Here it is surrounded by a small quantity of protoplasm, and with this forms the lens-shaped formative yelk (Fig. 15 b). This is seen on the yellow yelk-ball, at a certain point of the surface, as a small round white spot—the “tread” (cicatricula). From this point a thread-like column of white nutritive yelk (d), which contains no yellow yelk-granules, and is softer than the yellow food-yelk, proceeds to the middle of the yellow yelk-ball, and forms there a small central globule of white yelk (Fig. 15 d). The whole of this white yelk is not sharply separated from the yellow yelk, which shows a slight trace of concentric layers in the hard-boiled egg (Fig. 15 c). We also find in the hen’s egg, when we break the shell and take out the yelk, a round small white disk at its surface which corresponds to the tread. But this small white “germinal disk” is now further developed, and is really the gastrula of the chick. The body of the chick is formed from it alone. The whole white and yellow yelk-mass is without any significance for the formation of the embryo, it being merely used as food by the developing chick. The clear, glarous mass of albumin that surrounds the yellow yelk of the bird’s egg, and also the hard chalky shell, are only formed within the oviduct round the impregnated ovum.
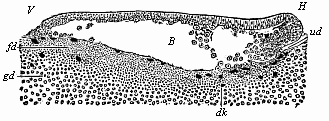
Fig. 56—Longitudinal section of the blastula of a shark (Pristiurus) at the beginning of gastrulation. (From Ruckert.) (Seen from the left.) V fore end, H hind end, B segmentation-cavity, ud first trace of the primitive gut, dk yelk-nuclei, fd fine-grained yelk, gd coarse-grained yelk.
When the fertilisation of the bird’s ovum has taken place within the mother’s body, we find in the lens-shaped stem-cell the progress of flat, discoid segmentation (Fig. 57). First two equal segmentation-cells (A) are formed from the ovum. These divide into four (B), then into eight, sixteen (C), thirty-two, sixty-four, and so on. The cleavage of the cells is always preceded by a division of their nuclei. The cleavage surfaces between the segmentation-cells appear at the free surface of the tread as clefts. The first two divisions are vertical to each other, in the form of a cross (B). Then there are two more divisions, which cut the former at an angle of forty-five degrees. The tread, which thus becomes the germinal disk, now has the appearance of an eight-rayed star. A circular cleavage next taking place round the middle, the eight triangular cells divide into sixteen, of which eight are in the middle and eight distributed around (C). Afterwards circular clefts and radial clefts, directed towards the centre, alternate more or less irregularly (D, E). In most of the amniotes the formation of concentric and radial clefts is irregular from the very first; and so also in the hen’s egg. But the final outcome of the cleavage-process is once more the formation of a large number of small cells of a similar nature. As in the case of the fish-ovum, these segmentation-cells form a round, lens-shaped disk, which corresponds to the morula, and is embedded in a small depression of the white yelk. Between the lens-shaped disk of the morula-cells and the underlying white yelk a small cavity is now formed by the accumulation of fluid, as in the fishes. Thus we get the peculiar and not easily recognisable blastula of the bird (Fig. 58). The small segmentation-cavity (fh) is very flat and much compressed. The upper or dorsal wall (dw) is formed of a single layer of clear, distinctly separated cells; this corresponds to the upper or animal hemisphere of the triton-blastula (Fig. 45). The lower or ventral wall of the flat dividing space (vw) is made up of larger and darker segmentation-cells; it corresponds to the lower or vegetal hemisphere of the blastula of the water-salamander (Fig. 45 dz). The nuclei of the yelk-cells, which are in this case especially numerous at the edge of the lens-shaped blastula, travel into the white yelk, increase by cleavage, and contribute even to the further growth of the germinal disk by furnishing it with food-stuff.
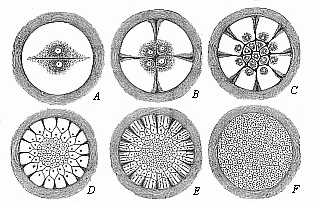
Fig. 57—Diagram of discoid segmentation in the bird’s ovum (magnified). Only the formative yelk (the tread) is shown in these six figures (A to F), because cleavage only takes place in this. The much larger food-yelk, which does not share in the cleavage, is left out and merely indicated by the dark ring without.
The invagination or the folding inwards of the bird-blastula takes place in this case also at the hinder pole of the subsequent chief axis, in the middle of the hind border of the round germinal disk (Fig. 59 s). At this spot we have the most brisk cleavage of the cells; hence the cells are more numerous and smaller here than in the fore-half of the germinal disk. The border-swelling or thick edge of the disk is less clear but whiter behind, and is more sharply separated from contiguous parts. In the middle of its hind border there is a white, crescent-shaped groove—Koller’s sickle-groove (Fig. 59 s); a small projecting process in the centre of it is called the sickle-knob (sk). This important cleft is the primitive mouth, which was described for a long time as the “primitive groove.” If we make a vertical section through this part, we see that a flat and broad cleft stretches under the germinal disk forwards from the primitive mouth; this is the primitive gut (Fig. 60 ud). Its roof or dorsal wall is formed by the folded upper part of the blastula, and its floor or ventral wall by the white yelk (wd), in which a number of yelk-nuclei (dk) are distributed. There is a brisk multiplication of these at the edge of the germinal disk, especially in the neighbourhood of the sickle-shaped primitive mouth.
We learn from sections through later stages of this discoid bird-gastrula that the primitive gut-cavity, extending forward from the primitive mouth as a flat pouch, undermines the whole region of the round flat lens-shaped blastula (Fig. 61 ud). At the same time, the segmentation-cavity gradually disappears altogether, the folded inner germinal layer (ik) placing itself from underneath on the overlying outer germinal layer (ak). The typical process of invagination, though greatly disguised, can thus be clearly seen in this case, as Goette and Rauber, and more recently Duval (Fig. 61), have shown.
The older embryologists (Pander, Baer, Remak), and, in recent times especially, His, Kölliker, and others, said that the two primary germinal layers of the hen’s ovum—the oldest and most frequent subject of observation!—arose by horizontal cleavage of a simple germinal disk. In opposition to this accepted view, I affirmed in my Gastræa Theory (1873) that the discoid bird-gastrula, like that of all other vertebrates, is formed by folding (or invagination), and that this typical process is merely altered in a peculiar way and disguised by the immense accumulation of food-yelk and the flat spreading of the discoid blastula at one part of its surface. I endeavoured to establish this view by the derivation of the vertebrates from one source, and especially by proving that the birds descend from the reptiles, and these from the amphibia. If this is correct, the discoid gastrula of the amniotes must have been formed by the folding-in of a hollow blastula, as has been shown by Remak and Rusconi of the discoid gastrula of the amphibia, their direct ancestors. The accurate and extremely careful observations of the authors I have mentioned (Goette, Rauber, and Duval) have decisively proved this recently for the birds; and the same has been done for the reptiles by the fine studies of Kupffer, Beneke, Wenkebach, and others. In the shield-shaped germinal disk of the lizard (Fig. 62), the crocodile, the tortoise, and other reptiles, we find in the middle of the hind border (at the same spot as the sickle groove in the bird) a transverse furrow (u), which leads into a flat, pouch-like, blind sac, the primitive gut. The fore (dorsal) and hind (ventral) lips of the transverse furrow correspond exactly to the lips of the primitive mouth (or sickle-groove) in the birds.
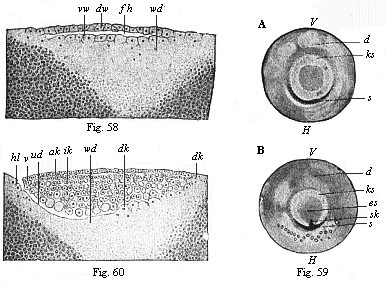
Fig.
58—Vertical section of the blastula of a hen
(discoblastula). fh segmentation-cavity, dw dorsal wall
of same, vw ventral wall, passing directly into the white yelk
(wd). (From Duval.)
Fig. 59—The germinal disk of
the hen’s ovum at the beginning of gastrulation; A before
incubation, B in the first hour of incubation. (From Koller.)
ks germinal-disk, V its fore and H its hind border;
es embryonic shield, s sickle-groove, sk sickle knob,
d yelk.
Fig. 60—Longitudinal section of the germinal disk
of a siskin (discogastrula). (From Duval.) ud
primitive gut, vl, hl fore and hind lips of the primitive mouth (or
sickle-edge); ak outer germinal layer, ik inner germinal layer,
dk yelk-nuclei, wd white yelk.

Fig. 61—Longitudinal section of the discoid gastrula of the nightingale. (From Duval.) ud primitive gut, vl, hl fore and hind lips of the primitive mouth; ak, ik outer and inner germinal layers; vr fore-border of the discogastrula.
Fig. 62—Germinal disk of the lizard (Lacerta agilis). (From Kupffer.) u primitive mouth, s sickle, es embryonic shield, hf and df light and dark germinative area.
The gastrulation of the mammals must be derived from this special embryonic development of the reptiles and birds. This latest and most advanced class of the vertebrates has, as we shall see afterwards, evolved at a comparatively recent date from an older group of reptiles; and all these amniotes must have come originally from a common stem-form. Hence the distinctive embryonic process of the mammal must have arisen by cenogenetic modifications from the older form of gastrulation of the reptiles and birds. Until we admit this thesis we cannot understand the formation of the germinal layers in the mammal, and therefore in man.
I first advanced this fundamental principle in my essay On the Gastrulation of Mammals (1877), and sought to show in this way that I assumed a gradual degeneration of the food-yelk and the yelk-sac on the way from the proreptiles to the mammals. “The cenogenetic process of adaptation,” I said, “which has occasioned the atrophy of the rudimentary yelk-sac of the mammal, is perfectly clear. It is due to the fact that the young of the mammal, whose ancestors were certainly oviparous, now remain a long time in the womb. As the great store of food-yelk, which the oviparous ancestors gave to the egg, became superfluous in their descendants owing to the long carrying in the womb, and the maternal blood in the wall of the uterus made itself the chief source of nourishment, the now useless yelk-sac was bound to atrophy by embryonic adaptation.”
My opinion met with little approval at the time; it was vehemently attacked by Kölliker, Hensen, and His in particular. However, it has been gradually accepted, and has recently been firmly established by a large number of excellent studies of mammal gastrulation, especially by Edward Van Beneden’s studies of the rabbit and bat, Selenka’s on the marsupials and rodents, Heape’s and Lieberkühn’s on the mole, Kupffer and Keibel’s on the rodents, Bonnet’s on the ruminants, etc. From the general comparative point of view, Carl Rabl in his theory of the mesoderm, Oscar Hertwig in the latest edition of his Manual (1902), and Hubrecht in his Studies in Mammalian Embryology (1891), have supported the opinion, and sought to derive the peculiarly modified gastrulation of the mammal from that of the reptile.
In the meantime (1884) the studies of Wilhelm Haacke and Caldwell provided a proof of the long-suspected and very interesting fact, that the lowest mammals, the monotremes, lay eggs, like the birds and reptiles, and are not viviparous like the other mammals. Although the gastrulation of the monotremes was not really known until studied by Richard Semon in 1894, there could be little doubt, in view of the great size of their food-yelk, that their ovum-segmentation was discoid, and led to the formation of a sickle-mouthed discogastrula, as in the case of the reptiles and birds. Hence I had, in 1875 (in my essay on The Gastrula and Ovum-segmentation of Animals), counted the monotremes among the discoblastic vertebrates. This hypothesis was established as a fact nineteen years afterwards by the careful observations of Semon; he gave in the second volume of his great work, Zoological Journeys in Australia (1894), the first description and correct explanation of the discoid gastrulation of the monotremes. The fertilised ova of the two living monotremes (Echidna and Ornithorhynchus) are balls of one-fifth of an inch in diameter, enclosed in a stiff shell; but they grow considerably during development, so that when laid the egg is three times as large. The structure of the plentiful yelk, and especially the relation of the yellow and the white yelk, are just the same as in the reptiles and birds. As with these, partial cleavage takes place at a spot on the surface at which the small formative yelk and the nucleus it encloses are found. First is formed a lens-shaped circular germinal disk. This is made up of several strata of cells, but it spreads over the yelk-ball, and thus becomes a one-layered blastula.
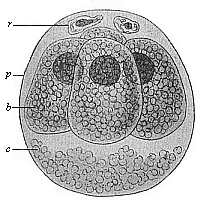
Fig. 63—Ovum of the opossum (Didelphys) divided into four. (From Selenka.) b the four segmentation-cells, r directive body, c unnucleated coagulated matter, p, albumin-membrane.
If we then imagine the yelk it contains to be dissolved and replaced by a clear liquid, we have the characteristic blastula of the higher mammals. In these the gastrulation proceeds in two phases, as Semon rightly observes: firstly, formation of the entoderm by cleavage at the centre and further growth at the edge; secondly, invagination. In the monotremes more primitive conditions have been retained better than in the reptiles and birds. In the latter, before the commencement of the gastrula-folding, we have, at least at the periphery, a two-layered embryo forming from the cleavage. But in the monotremes the formation of the cenogenetic entoderm does not precede the invagination; hence in this case the construction of the germinal layers is less modified than in the other amniota.
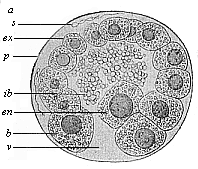
Fig. 64—Blastula of the opossum (Didelphys). (From Selenka.) a animal pole of the blastula, v vegetal pole, en mother-cell of the entoderm, ex ectodermic cells, s spermia, ib unnucleated yelk-balls (remainder of the food-yelk), p albumin membrane.
The marsupials, a second sub-class, come next to the oviparous monotremes, the oldest of the mammals. But as in their case the food-yelk is already atrophied, and the little ovum develops within the mother’s body, the partial cleavage has been reconverted into total. One section of the marsupials still show points of agreement with the monotremes, while another section of them, according to the splendid investigations of Selenka, form a connecting-link between these and the placentals.
The fertilised ovum of the opossum (Didelphys) divides, according to Selenka, first into two, then four, then eight equal cells; hence the segmentation is at first equal or homogeneous. But in the course of the cleavage a larger cell, distinguished by its less clear plasm and its containing more yelk-granules (the mother cell of the entoderm, Fig. 64 en), separates from the others; the latter multiply more rapidly than the former. As, further, a quantity of fluid gathers in the morula, we get a round blastula, the wall of which is of varying thickness, like that of the amphioxus (Fig. 38 E) and the amphibia (Fig. 45). The upper or animal hemisphere is formed of a large number of small cells; the lower or vegetal hemisphere of a small number of large cells. One of the latter, distinguished by its size (Fig. 64 en), lies at the vegetal pole of the blastula-axis, at the point where the primitive mouth afterwards appears. This is the mother-cell of the entoderm; it now begins to multiply by cleavage, and the daughter-cells (Fig. 65 i) spread out from this spot over the inner surface of the blastula, though at first only over the vegetal hemisphere. The less clear entodermic cells (i) are distinguished at first by their rounder shape and darker nuclei from the higher, clearer, and longer entodermic cells (e), afterwards both are greatly flattened, the inner blastodermic cells more than the outer.
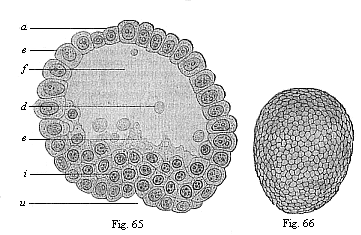
Fig. 65—Blastula of the opossum
(Didelphys) at the beginning of gastrulation. (From Selenka.)
e ectoderm, i entoderm; a animal pole, u primitive
mouth at the vegetal pole, f segmentation-cavity, d unnucleated
yelk-balls (relics of the reduced food-yelk), c nucleated curd (without
yelk-granules)
Fig. 66—Oval gastrula of the opossum
(Didelphys), about eight hours old. (From Selenka) (external
view).)
The unnucleated yelk-balls and curd (Fig. 65 d) that we find in the fluid of the blastula in these marsupials are very remarkable; they are the relics of the atrophied food-yelk, which was developed in their ancestors, the monotremes, and in the reptiles.
In the further course of the gastrulation of the opossum the oval shape of the gastrula (Fig. 66) gradually changes into globular, a larger quantity of fluid accumulating in the vesicle. At the same time, the entoderm spreads further and further over the inner surface of the ectoderm (e). A globular vesicle is formed, the wall of which consists of two thin simple strata of cells; the cells of the outer germinal layer are rounder, and those of the inner layer flatter. In the region of the primitive mouth (p) the cells are less flattened, and multiply briskly. From this point—from the hind (ventral) lip of the primitive mouth, which extends in a central cleft, the primitive groove—the construction of the mesoderm proceeds.
Gastrulation is still more modified and curtailed cenogenetically in the placentals than in the marsupials. It was first accurately known to us by the distinguished investigations of Edward Van Beneden in 1875, the first object of study being the ovum of the rabbit. But as man also belongs to this sub-class, and as his as yet unstudied gastrulation cannot be materially different from that of the other placentals, it merits the closest attention. We have, in the first place, the peculiar feature that the two first segmentation-cells that proceed from the cleavage of the fertilised ovum (Fig. 68) are of different sizes and natures; the difference is sometimes greater, sometimes less (Fig. 69). One of these first daughter-cells of the ovum is a little larger, clearer, and more transparent than the other. Further, the smaller cell takes a colour in carmine, osmium, etc., more strongly than the larger. By repeated cleavage of it a morula is formed, and from this a blastula, which changes in a very characteristic way into the greatly modified gastrula. When the number of the segmentation-cells in the mammal embryo has reached ninety-six (in the rabbit, about seventy hours after impregnation) the fœtus assumes a form very like the archigastrula (Fig. 72). The spherical embryo consists of a central mass of thirty-two soft, round cells with dark nuclei, which are flattened into polygonal shape by mutual pressure, and colour dark-brown with osmic acid (Fig. 72 i). This dark central group of cells is surrounded by a lighter spherical membrane, consisting of sixty-four cube-shaped, small, and fine-grained cells which lie close together in a single stratum, and only colour slightly in osmic acid (Fig. 72 e). The authors who regard this embryonic form as the primary gastrula of the placental conceive the outer layer as the ectoderm and the inner as the entoderm. The entodermic membrane is only interrupted at one spot, one, two, or three of the ectodermic cells being loose there. These form the yelk-stopper, and fill up the mouth of the gastrula (a). The central primitive gut-cavity (d) is full of entodermic cells. The uni-axial type of the mammal gastrula is accentuated in this way. However, opinions still differ considerably as to the real nature of this “provisional gastrula” of the placental and its relation to the blastula into which it is converted.
As the gastrulation proceeds a large spherical blastula is formed from this peculiar solid amphigastrula of the placental, as we saw in the case of the marsupial. The accumulation of fluid in the solid gastrula (Fig. 73 A) leads to the formation of an eccentric cavity, the group of the darker entodermic cells (hy) remaining directly attached at one spot with the round enveloping stratum of the lighter ectodermic cells (ep). This spot corresponds to the original primitive mouth (prostoma or blastoporus). From this important spot the inner germinal layer spreads all round on the inner surface of the outer layer, the cell-stratum of which forms the wall of the hollow sphere; the extension proceeds from the vegetal towards the animal pole.
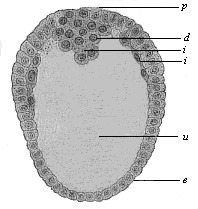
Fig. 67—Longitudinal section through the oval gastrula of the opossum (Fig. 69). (From Selenka.) p primitive mouth, e ectoderm, i entoderm, d yelk remains in the primitive gut-cavity (u).
The cenogenetic gastrulation of the placental has been greatly modified by secondary adaptation in the various groups of this most advanced and youngest sub-class of the mammals. Thus, for instance, we find in many of the rodents (guinea-pigs, mice, etc.) apparently a temporary inversion of the two germinal layers. This is due to a folding of the blastodermic wall by what is called the “girder,” a plug-shaped growth of Rauber’s “roof-layer.” It is a thin layer of flat epithelial cells, that is freed from the surface of the blastoderm in some of the rodents; it has no more significance in connection with the general course of placental gastrulation than the conspicuous departure from the usual globular shape in the blastula of some of the ungulates. In some pigs and ruminants it grows into a thread-like, long and thin tube.
Thus the gastrulation of the placentals, which diverges most from that of the amphioxus, the primitive form, is reduced to the original type, the invagination of a modified blastula. Its chief peculiarity is that the folded part of the blastoderm does not form a completely closed (only open at the primitive mouth) blind sac, as is usual; but this blind sac has a wide opening at the ventral curve (opposite to the dorsal mouth); and through this opening the primitive gut communicates from the first with the embryonic cavity of the blastula. The folded crest-shaped entoderm grows with a free circular border on the inner surface of the entoderm towards the vegetal pole; when it has reached this, and the inner surface of the blastula is completely grown over, the primitive gut is closed. This remarkable direct transition of the primitive gut-cavity into the segmentation-cavity is explained simply by the assumption that in most of the mammals the yelk-mass, which is still possessed by the oldest forms of the class (the monotremes) and their ancestors (the reptiles), is atrophied. This proves the essential unity of gastrulation in all the vertebrates, in spite of the striking differences in the various classes.
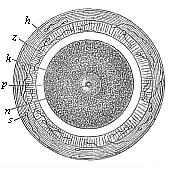
Fig. 68—Stem-cell of the mammal ovum (from the
rabbit).
k stem-nucleus, n nuclear corpuscle, p
protoplasm of the stem-cell, z modified zona pellucida, h
outer albuminous membrane, s dead sperm-cells.
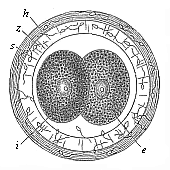
Fig. 69—Incipient cleavage of the mammal ovum (from the rabbit). The stem-cell has divided into two unequal cells, one lighter (e) and one darker (i). z zona pellucida, h outer albuminous membrane, s dead sperm-cell.
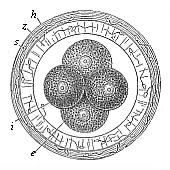
Fig. 70—The first four
segmentation-cells of the mammal ovum (from the rabbit).
e the
two larger (and lighter) cells, i the two smaller (and darker)
cells, z zona pellucida, h outer albuminous membrane.
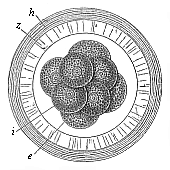
Fig. 71—Mammal ovum with eight segmentation-cells (from the rabbit). e four larger and lighter cells, i four smaller and darker cells, z zona pellucida, h outer albuminous membrane.
In order to complete our consideration of the important processes of segmentation and gastrulation, we will, in conclusion, cast a brief glance at the fourth chief type—superficial segmentation. In the vertebrates this form is not found at all. But it plays the chief part in the large stem of the articulates—the insects, spiders, myriapods, and crabs. The distinctive form of gastrula that comes of it is the “vesicular gastrula” (Perigastrula).
In the ova which undergo this superficial cleavage the formative yelk is sharply divided from the nutritive yelk, as in the preceding cases of the ova of birds, reptiles, fishes, etc.; the formative yelk alone undergoes cleavage. But while in the ova with discoid gastrulation the formative yelk is not in the centre, but at one pole of the uni-axial ovum, and the food-yelk gathered at the other pole, in the ova with superficial cleavage we find the formative yelk spread over the whole surface of the ovum; it encloses spherically the food-yelk, which is accumulated in the middle of the ova. As the segmentation only affects the former and not the latter, it is bound to be entirely “superficial”; the store of food in the middle is quite untouched by it. As a rule, it proceeds in regular geometrical progression. In the end the whole of the formative yelk divides into a number of small and homogeneous cells, which lie close together in a single stratum on the entire surface of the ovum, and form a superficial blastoderm. This blastoderm is a simple, completely closed vesicle, the internal cavity of which is entirely full of food-yelk. This real blastula only differs from that of the primitive ova in its chemical composition. In the latter the content is water or a watery jelly; in the former it is a thick mixture, rich in food-yelk, of albuminous and fatty substances. As this quantity of food-yelk fills the centre of the ovum before cleavage begins, there is no difference in this respect between the morula and the blastula. The two stages rather agree in this.
When the blastula is fully formed, we have again in this case the important folding or invagination that determines gastrulation. The space between the skin-layer and the gut-layer (the remainder of the segmentation-cavity) remains full of food-yelk, which is gradually used up. This is the only material difference between our vesicular gastrula (perigastrula) and the original form of the bell-gastrula (archigastrula). Clearly the one has been developed from the other in the course of time, owing to the accumulation of food-yelk in the centre of the ovum.[23]
[23] On the reduction of all forms of gastrulation to the original palingenetic form see especially the lucid treatment of the subject in Arnold Lang’s Manual of Comparative Anatomy (1888), Part I.
We must count it an important advance that we are thus in a position to reduce all the various embryonic phenomena in the different groups of animals to these four principal forms of segmentation and gastrulation. Of these four forms we must regard one only as the original palingenetic, and the other three as cenogenetic and derivative. The unequal, the discoid, and the superficial segmentation have all clearly arisen by secondary adaptation from the primary segmentation; and the chief cause of their development has been the gradual formation of the food-yelk, and the increasing antithesis between animal and vegetal halves of the ovum, or between ectoderm (skin-layer) and entoderm (gut-layer).
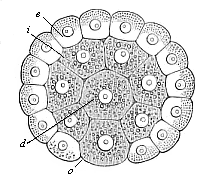
Fig. 72—Gastrula of the placental mammal (epigastrula from the rabbit), longitudinal section through the axis. e ectodermic cells (sixty-four, lighter and smaller), i entodermic cells (thirty-two, darker and larger), d central entodermic cell, filling the primitive gut-cavity, o peripheral entodermic cell, stopping up the opening of the primitive mouth (yelk-stopper in the Rusconian anus).
The numbers of careful studies of animal gastrulation that have been made in the last few decades have completely established the views I have expounded, and which I first advanced in the years 1872–76. For a time they were greatly disputed by many embryologists. Some said that the original embryonic form of the metazoa was not the gastrula, but the “planula”—a double-walled vesicle with closed cavity and without mouth-aperture; the latter was supposed to pierce through gradually. It was afterwards shown that this planula (found in several sponges, etc.) was a later evolution from the gastrula.
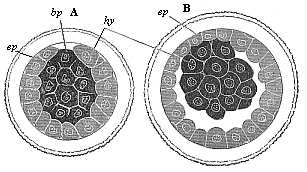
Fig. 73—Gastrula of the rabbit. A as a solid, spherical cluster of cells, B changing into the embryonic vesicle, bp primitive mouth, ep ectoderm, hy entoderm.
It was also shown that what is called delamination—the rise of the two primary germinal layers by the folding of the surface of the blastoderm (for instance, in the Geryonidæ and other medusæ)—was a secondary formation, due to cenogenetic variations from the original invagination of the blastula. The same may be said of what is called “immigration,” in which certain cells or groups of cells are detached from the simple layer of the blastoderm, and travel into the interior of the blastula; they attach themselves to the inner wall of the blastula, and form a second internal epithelial layer—that is to say, the entoderm. In these and many other controversies of modern embryology the first requisite for clear and natural explanation is a careful and discriminative distinction between palingenetic (hereditary) and cenogenetic (adaptive) processes. If this is properly attended to, we find evidence everywhere of the biogenetic law.
The two “primary germinal layers” which the gastræa theory has shown to be the first foundation in the construction of the body are found in this simplest form throughout life only in animals of the lowest grade—in the gastræads, olynthus (the stem-form of the sponges), hydra, and similar very simple animals. In all the other animals new strata of cells are formed subsequently between these two primary body-layers, and these are generally comprehended under the title of the middle layer, or mesoderm. As a rule, the various products of this middle layer afterwards constitute the great bulk of the animal frame, while the original entoderm, or internal germinal layer, is restricted to the clothing of the alimentary canal and its glandular appendages; and, on the other hand, the ectoderm, or external germinal layer, furnishes the outer clothing of the body, the skin and nervous system.
In some large groups of the lower animals, such as the sponges, corals, and flat-worms, the middle germinal layer remains a single connected mass, and most of the body is developed from it; these have been called the three-layered metazoa, in opposition to the two-layered animals described. Like the two-layered animals, they have no body-cavity—that is to say, no cavity distinct from the alimentary system. On the other hand, all the higher animals have this real body-cavity (cœloma), and so are called cœlomaria. In all these we can distinguish four secondary germinal layers, which develop from the two primary layers. To the same class belong all true vermalia (excepting the platodes), and also the higher typical animal stems that have been evolved from them—molluscs, echinoderms, articulates, tunicates, and vertebrates.
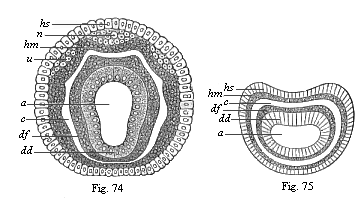
Figs. 74 and 75—Diagram of the four secondary germinal layers, transverse section through the metazoic embryo: Fig. 74 of an annelid, Fig. 75 of a vermalian. a primitive gut, dd ventral glandular layer, df ventral fibre-layer, hm skin-fibre-layer, hs skin-sense-layer, u beginning of the rudimentary kidneys, n beginning of the nerve-plates.
The body-cavity (cœloma) is therefore a new acquisition of the animal body, much younger than the alimentary system, and of great importance. I first pointed out this fundamental significance of the cœlom in my Monograph on the Sponges (1872), in the section which draws a distinction between the body-cavity and the gut-cavity, and which follows immediately on the germ-layer theory and the ancestral tree of the animal kingdom (the first sketch of the gastræa theory). Up to that time these two principal cavities of the animal body had been confused, or very imperfectly distinguished; chiefly because Leuckart, the founder of the cœlenterata group (1848), has attributed a body-cavity, but not a gut-cavity, to these lowest metazoa. In reality, the truth is just the other way about.
The ventral cavity, the original organ of nutrition in the multicellular animal-body, is the oldest and most important organ of all the metazoa, and, together with the primitive mouth, is formed in every case in the gastrula as the primitive gut; it is only at a much later stage that the body-cavity, which is entirely wanting in the cœlenterata, is developed in some of the metazoa between the ventral and the body wall. The two cavities are entirely different in content and purport. The alimentary cavity (enteron) serves the purpose of digestion; it contains water and food taken from without, as well as the pulp (chymus) formed from this by digestion. On the other hand, the body-cavity, quite distinct from the gut and closed externally, has nothing to do with digestion; it encloses the gut itself and its glandular appendages, and also contains the sexual products and a certain amount of blood or lymph, a fluid that is transuded through the ventral wall.
As soon as the body-cavity appears, the ventral wall is found to be separated from the enclosing body-wall, but the two continue to be directly connected at various points. We can also then always distinguish a number of different layers of tissue in both walls—at least two in each. These tissue-layers are formed originally from four different simple cell-layers, which are the much-discussed four secondary germinal layers. The outermost of these, the skin-sense-layer (Figs. 74, 75 hs), and the innermost, the gut-gland-layer (dd), remain at first simple epithelia or covering-layers. The one covers the outer surface of the body, the other the inner surface of the ventral wall; hence they are called confining or limiting layers. Between them are the two middle-layers, or mesoblasts, which enclose the body-cavity.
Fig. 76—Cœlomula of sagitta (gastrula with a couple of cœlom-pouches. (From Kowalevsky.) bl.p primitive mouth, al primitive gut, pv cœlom-folds, m permanent mouth.
The four secondary germinal layers are so distributed in the structure of the body in all the cœlomaria (or all metazoa that have a body-cavity) that the outer two, joined fast together, constitute the body-wall, and the inner two the ventral wall; the two walls are separated by the cavity of the cœlom. Each of the walls is made up of a limiting layer and a middle layer. The two limiting layers chiefly give rise to epithelia, or covering-tissues, and glands and nerves, while the middle layers form the great bulk of the fibrous tissue, muscles, and connective matter. Hence the latter have also been called fibrous or muscular layers. The outer middle layer, which lies on the inner side of the skin-sense-layer, is the skin fibre-layer; the inner middle layer, which attaches from without to the ventral glandular layer, is the ventral fibre layer. The former is usually called briefly the parietal, and the latter the visceral layer or mesoderm. Of the many different names that have been given to the four secondary germinal layers, the following are those most in use to-day:—
| 1. Skin-sense-layer (outer limiting layer). | I. Neural layer (neuroblast). | The two secondary germinal layers of the body-wall: I. Epithelial. II. Fibrous. |
| 2. Skin-fibre-layer (outer middle layer). | II. Parietal layer (myoblast). | |
| 3. Gut-fibre-layer (inner middle layer). | III. Visceral layer (genoblast). | The two secondary germinal layers of the gut-wall: III. Fibrous. IV. Epithelial. |
| 4. Gut-gland-layer (inner limiting layer). | IV. Enteral layer (enteroblast) |
The first scientist to recognise and clearly distinguish the four secondary germinal layers was Baer. It is true that he was not quite clear as to their origin and further significance, and made several mistakes in detail in explaining them. But, on the whole, their great importance did not escape him. However, in later years his view had to be given up in consequence of more accurate observations. Remak then propounded a three-layer theory, which was generally accepted. These theories of cleavage, however, began to give way thirty years ago, when Kowalevsky (1871) showed that in the case of Sagitta (a very clear and typical subject of gastrulation) the two middle germinal layers and the two limiting layers arise not by cleavage, but by folding—by a secondary invagination of the primary inner germ-layer. This invagination or folding proceeds from the primitive mouth, at the two sides of which (right and left) a couple of pouches are formed. As these cœlom-pouches or cœlom-sacs detach themselves from the primitive gut, a double body-cavity is formed (Figs. 74–76).
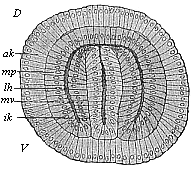
Fig. 77—Cœlomula of sagitta, in section. (From Hertwig.) D dorsal side, V ventral side, ik inner germinal layer, mv visceral mesoblast, lh body-cavity, mp parietal mesoblast, ak outer germinal layer.
The same kind of cœlom-formation as in sagitta was afterwards found by Kowalevsky in brachiopods and other invertebrates, and in the lowest vertebrate—the amphioxus. Further instances were discovered by two English embryologists, to whom we owe very considerable advance in ontogeny—E. Ray-Lankester and F. Balfour. On the strength of these and other studies, as well as most extensive research of their own, the brothers Oscar and Richard Hertwig constructed in 1881 the Cœlom Theory. In order to appreciate fully the great merit of this illuminating and helpful theory, one must remember what a chaos of contradictory views was then represented by the “problem of the mesoderm,” or the much-disputed “question of the origin of the middle germinal layer.” The cœlom theory brought some light and order into this infinite confusion by establishing the following points: 1. The body-cavity originates in the great majority of animals (especially in all the vertebrates) in the same way as in sagitta: a couple of pouches or sacs are formed by folding inwards at the primitive mouth, between the two primary germinal layers; as these pouches detach from the primitive gut, a pair of cœlom-sacs (right and left) are formed; the coalescence of these produces a simple body-cavity. 2. When these cœlom-embryos develop, not as a pair of hollow pouches, but as solid layers of cells (in the shape of a pair of mesodermal streaks)—as happens in the higher vertebrates—we have a secondary (cenogenetic) modification of the primary (palingenetic) structure; the two walls of the pouches, inner and outer, have been pressed together by the expansion of the large food-yelk. 3. Hence the mesoderm consists from the first of two genetically distinct layers, which do not originate by the cleavage of a primary simple middle layer (as Remak supposed). 4. These two middle layers have, in all vertebrates, and the great majority of the invertebrates, the same radical significance for the construction of the animal body; the inner middle layer, or the visceral mesoderm, (gut-fibre layer), attaches itself to the original entoderm, and forms the fibrous, muscular, and connective part of the visceral wall; the outer middle layer, or the parietal mesoderm (skin-fibre-layer), attaches itself to the original ectoderm and forms the fibrous, muscular, and connective part of the body-wall. 5. It is only at the point of origination, the primitive mouth and its vicinity, that the four secondary germinal layers are directly connected; from this point the two middle layers advance forward separately between the two primary germinal layers, to which they severally attach themselves. 6. The further separation or differentiation of the four secondary germinal layers and their division into the various tissues and organs take place especially in the later fore-part or head of the embryo, and extend backwards from there towards the primitive mouth.
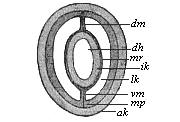
Fig. 78—Section of a young sagitta. (From Hertwig.) dh visceral cavity, ik and ak inner and outer limiting layers, mv and mp inner and outer middle layers, lk body-cavity, dm and vm dorsal and visceral mesentery.
All animals in which the body-cavity demonstrably arises in this way from the primitive gut (vertebrates, tunicates, echinoderms, articulates, and a part of the vermalia) were comprised by the Hertwigs under the title of enterocœla, and were contrasted with the other groups of the pseudocœla (with false body-cavity) and the cœlenterata (with no body-cavity). However, this radical distinction and the views as to classification which it occasioned have been shown to be untenable. Further, the absolute differences in tissue-formation which the Hertwigs set up between the enterocœla and pseudocœla cannot be sustained in this connection. For these and other reasons their cœlom-theory has been much criticised and partly abandoned. Nevertheless, it has rendered a great and lasting service in the solution of the difficult problem of the mesoderm, and a material part of it will certainly be retained. I consider it an especial merit of the theory that it has established the identity of the development of the two middle layers in all the vertebrates, and has traced them as cenogenetic modifications back to the original palingenetic form of development that we still find in the amphioxus. Carl Rabl comes to the same conclusion in his able Theory of the Mesoderm, and so do Ray-Lankester, Rauber, Kupffer, Ruckert, Selenka, Hatschek, and others. There is a general agreement in these and many other recent writers that all the different forms of cœlom-construction, like those of gastrulation, follow one and the same strict hereditary law in the vast vertebrate stem; in spite of their apparent differences, they are all only cenogenetic modifications of one palingenetic type, and this original type has been preserved for us down to the present day by the invaluable amphioxus.
But before we go into the regular cœlomation of the amphioxus, we will glance at that of the arrow-worm (Sagitta), a remarkable deep-sea worm that is interesting in many ways for comparative anatomy and ontogeny. On the one hand, the transparency of the body and the embryo, and, on the other hand, the typical simplicity of its embryonic development, make the sagitta a most instructive object in connection with various problems. The class of the chætogatha, which is only represented by the cognate genera of Sagitta and Spadella, is in another respect also a most remarkable branch of the extensive vermalia stem. It was therefore very gratifying that Oscar Hertwig (1880) fully explained the anatomy, classification, and evolution of the chætognatha in his careful monograph.
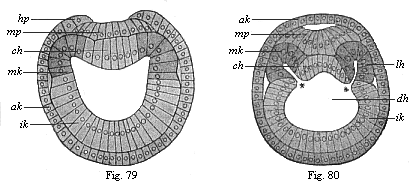
Figs. 79 and 80.—Transverse section of amphioxus-larvæ. (From Hatschek.) Fig. 79 at the commencement of cœlom formation (still without segments), Fig. 80 at the stage with four primitive segments. ak, ik, mk outer, inner, and middle germinal layer, hp horn plate, mp medullary plate, ch chorda, * and * disposition of the cœlom-pouches, lh body-cavity.)
The spherical blastula that arises from the impregnated ovum of the sagitta is converted by a folding at one pole into a typical archigastrula, entirely similar to that of the Monoxenia which I described (Chapter VIII, Fig. 29). This oval, uni-axial cup-larva (circular in section) becomes bilateral (or tri-axial) by the growth of a couple of cœlom-pouches from the primitive gut (Figs. 76, 77). To the right and left a sac-shaped fold appears towards the top pole (where the permanent mouth, m, afterwards arises). The two sacs are at first separated by a couple of folds of the entoderm (Fig. 76 pv), and are still connected with the primitive gut by wide apertures; they also communicate for a short time with the dorsal side (Fig. 77 d). Soon, however, the cœlom-pouches completely separate from each other and from the primitive gut; at the same time they enlarge so much that they close round the primitive gut (Fig. 78). But in the middle line of the dorsal and ventral sides the pouches remain separated, their approaching walls joining here to form a thin vertical partition, the mesentery (dm and vm). Thus Sagitta has throughout life a double body-cavity (Fig. 78 lk), and the gut is fastened to the body-wall both above and below by a mesentery—below by the ventral mesentery (vm), and above by the dorsal mesentery (dm). The inner layer of the two cœlom-pouches (mv) attaches itself to the entoderm (ik), and forms with it the visceral wall. The outer layer (mp) attaches itself to the ectoderm (ak), and forms with it the outer body-wall. Thus we have in Sagitta a perfectly clear and simple illustration of the original cœlomation of the enterocœla. This palingenetic fact is the more important, as the greater part of the two body-cavities in Sagitta changes afterwards into sexual glands—the fore or female part into a pair of ovaries, and the hind or male part into a pair of testicles.
Cœlomation takes place with equal clearness and transparency in the case of the amphioxus, the lowest vertebrate, and its nearest relatives, the invertebrate tunicates, the sea-squirts. However, in these two stems, which we class together as Chordonia, this important process is more complex, as two other processes are associated with it—the development of the chorda from the entoderm and the separation of the medullary plate or nervous centre from the ectoderm. Here again the skulless amphioxus has preserved to our own time by tenacious heredity the chief phenomena in their original form, while it has been more or less modified by embryonic adaptation in all the other vertebrates (with skulls). Hence we must once more thoroughly understand the palingenetic embryonic features of the lancelet before we go on to consider the cenogenetic forms of the craniota.
Figs. 81 and 82.—Transverse section of amphioxus embryo. Fig. 81 at the stage with five somites, Fig. 82 at the stage with eleven somites. (From Hatschek.) ak outer germinal layer, mp medullary plate, n nerve-tube, ik inner germinal layer, dh visceral cavity, lh body-cavity, mk middle germinal layer (mk1 parietal, mk2 visceral), us primitive segment, ch chorda.
The cœlomation of the amphioxus, which was first observed by Kowalevsky in 1867, has been very carefully studied since by Hatschek (1881). According to him, there are first formed on the bilateral gastrula we have already considered (Figs. 36, 37) three parallel longitudinal folds—one single ectodermal fold in the central line of the dorsal surface, and a pair of entodermic folds at the two sides of the former. The broad ectodermal fold that first appears in the middle line of the flattened dorsal surface, and forms a shallow longitudinal groove, is the beginning of the central nervous system, the medullary tube. Thus the primary outer germinal layer divides into two parts, the middle medullary plate (Fig. 81 mp) and the horny-plate (ak), the beginning of the outer skin or epidermis. As the parallel borders of the concave medullary plate fold towards each other and grow underneath the horny-plate, a cylindrical tube is formed, the medullary tube (Fig. 82 n); this quickly detaches itself altogether from the horny-plate. At each side of the medullary tube, between it and the alimentary tube (Figs. 79–82 dh), the two parallel longitudinal folds grow out of the dorsal wall of the alimentary tube, and these form the two cœlom-pouches (Figs. 80, 81 lh). This part of the entoderm, which thus represents the first structure of the middle germinal layer, is shown darker than the rest of the inner germinal layer in Figs. 79–82. The edges of the folds meet, and thus form closed tubes (Fig. 81 in section).
During this interesting process the outline of a third very important organ, the chorda or axial rod, is being formed between the two cœlom-pouches. This first foundation of the skeleton, a solid cylindrical cartilaginous rod, is formed in the middle line of the dorsal primitive gut-wall, from the entodermal cell-streak that remains here between the two cœlom-pouches (Figs. 79–82 ch). The chorda appears at first in the shape of a flat longitudinal fold or a shallow groove (Figs. 80, 81); it does not become a solid cylindrical cord until after separation from the primitive gut (Fig. 82). Hence we might say that the dorsal wall of the primitive gut forms three parallel longitudinal folds at this important period—one single fold and a pair of folds. The single middle fold becomes the chorda, and lies immediately below the groove of the ectoderm, which becomes the medullary tube; the pair of folds to the right and left lie at the sides between the former and the latter, and form the cœlom-pouches. The part of the primitive gut that remains after the cutting off of these three dorsal primitive organs is the permanent gut; its entoderm is the gut-gland-layer or enteric layer.
Figs. 83 and 84—Chordula of the amphioxus. Fig. 83 median longitudinal section (seen from the left). Fig. 84 transverse section. (From Hatschek.) In Fig. 83 the cœlom-pouches are omitted, in order to show the chordula more clearly. Fig. 84 is rather diagrammatic. h horny-plate, m medullary tube, n wall of same (n′ dorsal, n″ ventral), ch chorda, np neuroporus, ne canalis neurentericus, d gut-cavity, r gut dorsal wall, b gut ventral wall, z yelk-cells in the latter, u primitive mouth, o mouth-pit, p promesoblasts (primitive or polar cells of the mesoderm), w parietal layer, v visceral layer of the mesoderm, c cœlom, f rest of the segmentation-cavity.
Figs. 85 and 86—Chordula of the amphibia (the ringed adder). (From Goette.) Fig. 85 median longitudinal section (seen from the left), Fig. 86 transverse section (slightly diagrammatic). Lettering as in Figs. 83 and 84.
I give the name of chordula or chorda-larva to the embryonic stage of the vertebrate organism which is represented by the amphioxus larva at this period (Figs. 83, 84, in the third period of development according to Hatschek). (Strabo and Plinius give the name of cordula or cordyla to young fish larvæ.) I ascribe the utmost phylogenetic significance to it, as it is found in all the chorda-animals (tunicates as well as vertebrates) in essentially the same form. Although the accumulation of food-yelk greatly modifies the form of the chordula in the higher vertebrates, it remains the same in its main features throughout. In all cases the nerve-tube (m) lies on the dorsal side of the bilateral, worm-like body, the gut-tube (d) on the ventral side, the chorda (ch) between the two, on the long axis, and the cœlom pouches (c) at each side. In every case these primitive organs develop in the same way from the germinal layers, and the same organs always arise from them in the mature chorda-animal. Hence we may conclude, according to the laws of the theory of descent, that all these chordonia or chordata (tunicates and vertebrates) descend from an ancient common ancestral form, which we may call Chordæa. We should regard this long-extinct Chordæa, if it were still in existence, as a special class of unarticulated worm (chordaria). It is especially noteworthy that neither the dorsal nerve-tube nor the ventral gut-tube, nor even the chorda that lies between them, shows any trace of articulation or segmentation; even the two cœlom-sacs are not segmented at first (though in the amphioxus they quickly divide into a series of parts by transverse folding). These ontogenetic facts are of the greatest importance for the purpose of learning those ancestral forms of the vertebrates which we have to seek in the group of the unarticulated vermalia. The cœlom-pouches were originally sexual glands in these ancient chordonia.
Figs. 87 and 88—Diagrammatic vertical section of cœlomula-embryos of vertebrates. (From Hertwig.) Fig. 87, vertical section through the primitive mouth, Fig. 88, vertical section before the primitive mouth. u primitive mouth, ud primitive gut. d yelk, dk yelk-nuclei, dh gut-cavity, lh body-cavity, mp medullary plate, ch chorda plate, ak and ik outer and inner germinal layers, pb parietal and vb visceral mesoblast.
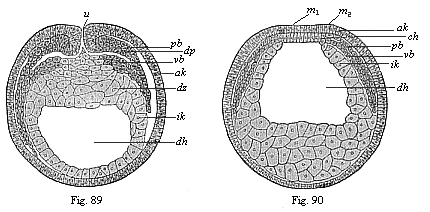
Figs. 89 and 90—Transverse section of cœlomula embryos of triton. (From Hertwig.) Fig. 89, section through the primitive mouth. Fig. 90, section in front of the primitive mouth, u primitive mouth. dh gut-cavity, dz yelk-cells, dp yelk-stopper, ak outer and ik inner germinal layer, pb parietal and vb visceral middle layer, m medullary plate, ch chorda.
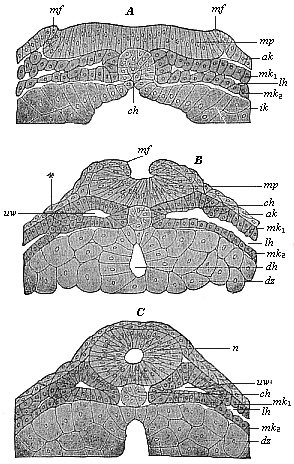
Fig. 91. A, B, C.—Vertical section of the dorsal part of three triton-embryos. (From Hertwig.) In Fig. A the medullary swellings (the parallel borders of the medullary plate) begin to rise; in Fig. B they grow towards each other; in Fig. C they join and form the medullary tube. mp medullary plate, mf medullary folds, n nerve-tube, ch chorda, lh body-cavity, mk1 and mk2 parietal and visceral mesoblasts, uv primitive-segment cavities, ak ectoderm, ik entoderm, dz yelk-cells, dh gut-cavity.
From the evolutionary point of view the cœlom-pouches are, in any case, older than the chorda; since they also develop in the same way as in the chordonia in a number of invertebrates which have no chorda (for instance, Sagitta, Figs. 76–78). Moreover, in the amphioxus the first outline of the chorda appears later than that of the cœlom-sacs. Hence we must, according to the biogenetic law, postulate a special intermediate form between the gastrula and the chordula, which we will call cœlomula, an unarticulated, worm-like body with primitive gut, primitive mouth, and a double body-cavity, but no chorda. This embryonic form, the bilateral cœlomula (Fig. 81), may in turn be regarded as the ontogenetic reproduction (maintained by heredity) of an ancient ancestral form of the cœlomaria, the Cœlomæa (cf. Chapter XX).
In Sagitta and other worm-like animals the two cœlom-pouches (presumably gonads or sex-glands) are separated by a complete median partition, the dorsal and ventral mesentery (Fig. 78 dm, vm); but in the vertebrates only the upper part of this vertical partition is maintained, and forms the dorsal mesentery. This mesentery afterwards takes the form of a thin membrane, which fastens the visceral tube to the chorda (or the vertebral column). At the under side of the visceral tube the cœlom-sacs blend together, their inner or median walls breaking down and disappearing. The body-cavity then forms a single simple hollow, in which the gut is quite free, or only attached to the dorsal wall by means of the mesentery.
The development of the body-cavity and the formation of the chordula in the higher vertebrates is, like that of the gastrula, chiefly modified by the pressure of the food-yelk on the embryonic structures, which forces its hinder part into a discoid expansion. These cenogenetic modifications seem to be so great that until twenty years ago these important processes were totally misunderstood. It was generally believed that the body-cavity in man and the higher vertebrates was due to the division of a simple middle layer, and that the latter arose by cleavage from one or both of the primary germinal layers. The truth was brought to light at last by the comparative embryological research of the Hertwigs. They showed in their Cœlom Theory (1881) that all vertebrates are true enterocœla, and that in every case a pair of cœlom-pouches are developed from the primitive gut by folding. The cenogenetic chordula-forms of the craniotes must therefore be derived from the palingenetic embryology of the amphioxus in the same way as I had previously proved for their gastrula-forms.
The chief difference between the cœlomation of the acrania (amphioxus) and the other vertebrates (with skulls—craniotes) is that the two cœlom-folds of the primitive gut in the former are from the first hollow vesicles, filled with fluid, but in the latter are empty pouches, the layers of which (inner and outer) close with each other. In common parlance we still call a pouch or pocket by that name, whether it is full or empty. It is different in ontogeny; in some of our embryological literature ordinary logic does not count for very much. In many of the manuals and large treatises on this science it is proved that vesicles, pouches, or sacs deserve that name only when they are inflated and filled with a clear fluid. When they are not so filled (for instance, when the primitive gut of the gastrula is filled with yelk, or when the walls of the empty cœlom-pouches are pressed together), these vesicles must not be cavities any longer, but “solid structures.”
The accumulation of food-yelk in the ventral wall of the primitive gut (Figs. 85, 86) is the simple cause that converts the sac-shaped cœlom-pouches of the acrania into the leaf-shaped cœlom-streaks of the craniotes. To convince ourselves of this we need only compare, with Hertwig, the palingenetic cœlomula of the amphioxus (Figs. 80, 81) with the corresponding cenogenetic form of the amphibia (Figs. 89–90), and construct the simple diagram that connects the two (Figs. 87, 88). If we imagine the ventral half of the primitive gut-wall in the amphioxus embryo (Figs. 79–84) distended with food-yelk, the vesicular cœlom-pouches (lh) must be pressed together by this, and forced to extend in the shape of a thin double plate between the gut-wall and body-wall (Figs. 86, 87). This expansion follows a downward and forward direction. They are not directly connected with these two walls. The real unbroken connection between the two middle layers and the primary germ-layers is found right at the back, in the region of the primitive mouth (Fig. 87 u). At this important spot we have the source of embryonic development (blastocrene), or “zone of growth,” from which the cœlomation (and also the gastrulation) originally proceeds.
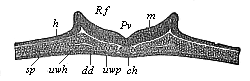
Fig. 92—Transverse section of the chordula-embryo of a bird (from a hen’s egg at the close of the first day of incubation). (From Kölliker.) h horn-plate (ectoderm), m medullary plate, Rf dorsal folds of same, Pv medullary furrow, ch chorda, uwp median (inner) part of the middle layer (median wall of the cœlom-pouches), sp lateral (outer) part of same, or lateral plates, uwh structure of the body-cavity, dd gut-gland-layer.
Hertwig even succeeded in showing, in the cœlomula-embryo of the water salamander (Triton), between the first structures of the two middle layers, the relic of the body-cavity, which is represented in the diagrammatic transitional form (Figs. 87, 88). In sections both through the primitive mouth itself (Fig. 89) and in front of it (Fig. 90) the two middle layers (pb and vb) diverge from each other, and disclose the two body-cavities as narrow clefts. At the primitive-mouth itself (Fig. 90 u) we can penetrate into them from without. It is only here at the border of the primitive mouth that we can show the direct transition of the two middle layers into the two limiting layers or primary germinal layers.
The structure of the chorda also shows the same features in these cœlomula-embryos of the amphibia (Fig. 91) as in the amphioxus (Figs. 79–82). It arises from the entodermic cell-streak, which forms the middle dorsal-line of the primitive gut, and occupies the space between the flat cœlom-pouches (Fig. 91 A). While the nervous centre is formed here in the middle line of the back and separated from the ectoderm as “medullary tube,” there takes place at the same time, directly underneath, the severance of the chorda from the entoderm (Fig. 91 A, B, C). Under the chorda is formed (out of the ventral entodermic half of the gastrula) the permanent gut or visceral cavity (enteron) (Fig. 91 B, dh). This is done by the coalescence, under the chorda in the median line, of the two dorsal side-borders of the gut-gland-layer (ik), which were previously separated by the chorda-plate (Fig. 91 A, ch); these now alone form the clothing of the visceral cavity (dh) (enteroderm, Fig. 91 C). All these important modifications take place at first in the fore or head-part of the embryo, and spread backwards from there; here at the hinder end, the region of the primitive mouth, the important border of the mouth (or properistoma) remains for a long time the source of development or the zone of fresh construction, in the further building-up of the organism. One has only to compare carefully the illustrations given (Figs. 85–91) to see that, as a fact, the cenogenetic cœlomation of the amphibia can be deduced directly from the palingenetic form of the acrania (Figs. 79–84).
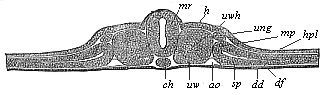
Fig. 93—Transverse section of the vertebrate-embryo of a bird (from a hen’s egg on the second day of incubation). (From Kölliker.) h horn-plate, mr medullary tube, ch chorda, uw primitive segments, uwh primitive-segment cavity (median relic of the cœlom), sp lateral cœlom-cleft, hpl skin-fibre-layer, df gut-fibre-layer, ung primitive-kidney passage, ao primitive aorta, dd gut-gland-layer.
The same principle holds good for the amniotes, the reptiles, birds, and mammals, although in this case the processes of cœlomation are more modified and more difficult to identify on account of the colossal accumulation of food-yelk and the corresponding notable flattening of the germinal disk. However, as the whole group of the amniotes has been developed at a comparatively late date from the class of the amphibia, their cœlomation must also be directly traceable to that of the latter. This is really possible as a matter of fact; even the older illustrations showed an essential identity of features. Thus forty years ago Kölliker gave, in the first edition of his Human Embryology (1861), some sections of the chicken-embryo, the features of which could at once be reduced to those already described and explained in the sense of Hertwig’s cœlom-theory. A section through the embryo in the hatched hen’s egg towards the close of the first day of incubation shows in the middle of the dorsal surface a broad ectodermic medullary groove (Fig. 92 Rf), and underneath the middle of the chorda (ch) and at each side of it a couple of broad mesodermic layers (sp). These enclose a narrow space or cleft (uwh), which is nothing else than the structure of the body-cavity. The two layers that enclose it—the upper parietal layer (hpl) and the lower visceral layer (df)—are pressed together from without, but clearly distinguishable. This is even clearer a little later, when the medullary furrow is closed into the nerve-tube (Fig. 93 mr).
Special importance attaches to the fact that here again the four secondary germinal layers are already sharply distinct, and easily separated from each other. There is only one very restricted area in which they are connected, and actually pass into each other; this is the region of the primitive mouth, which is contracted in the amniotes into a dorsal longitudinal cleft, the primitive groove. Its two lateral lip-borders form the primitive streak, which has long been recognised as the most important embryonic source and starting-point of further processes. Sections through this primitive streak (Figs. 94 and 95) show that the two primary germinal layers grow at an early stage (in the discoid gastrula of the chick, a few hours after incubation) into the primitive streak (x), and that the two middle layers extend outward from this thickened axial plate (y) to the right and left between the former. The plates of the cœlom-layers, the parietal skin-fibre-layer (m) and the visceral gut-fibre-layer (f), are seen to be still pressed close together, and only diverge later to form the body-cavity. Between the inner borders of the two flat cœlom-pouches lies the chorda (Fig. 95 x), which here again develops from the middle line of the dorsal wall of the primitive gut.
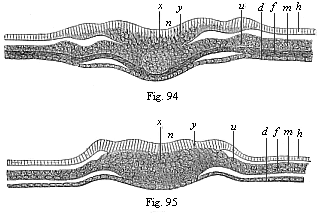
Figs. 94 and 95—Transverse section of the primitive-streak (primitive mouth) of the chick. Fig. 94 a few hours after the commencement of incubation, Fig. 95 a little later. (From Waldeyer.) h horn-plate, n nerve-plate, m skin-fibre-layer, f gut-fibre-layer, d gut-gland-layer, y primitive streak or axial plate, in which all four germinal layers meet, x structure of the chorda, u region of the later primitive kidneys.
Cœlomation takes place in the vertebrates in just the same way as in the birds and reptiles. This was to be expected, as the characteristic gastrulation of the mammal has descended from that of the reptiles. In both cases a discoid gastrula with primitive streak arises from the segmented ovum, a two-layered germinal disk with long and small hinder primitive mouth. Here again the two primary germinal layers are only directly connected (Fig. 96 pr) along the primitive streak (at the folding-point of the blastula), and from this spot (the border of the primitive mouth) the middle germinal layers (mk) grow out to right and left between the preceding. In the fine illustration of the cœlomula of the rabbit which Van Beneden has given us (Fig. 96) one can clearly see that each of the four secondary germinal layers consists of a single stratum of cells.
Finally, we must point out, as a fact of the utmost importance for our anthropogeny and of great general interest, that the four-layered cœlomula of man has just the same construction as that of the rabbit (Fig. 96). A vertical section that Count Spee made through the primitive mouth or streak of a very young human germinal disk (Fig. 97) clearly shows that here again the four secondary germ-layers are inseparably connected only at the primitive streak, and that here also the two flattened cœlom-pouches (mk) extend outwards to right and left from the primitive mouth between the outer and inner germinal layers. In this case, too, the middle germinal layer consists from the first of two separate strata of cells, the parietal (mp) and visceral (mv) mesoblasts.
These concordant results of the best recent investigations (which have been confirmed by the observations of a number of scientists I have not enumerated) prove the unity of the vertebrate-stem in point of cœlomation, no less than of gastrulation. In both respects the invaluable amphioxus—the sole survivor of the acrania—is found to be the original model that has preserved for us in palingenetic form by a tenacious heredity these most important embryonic processes. From this primary model of construction we can cenogenetically deduce all the embryonic forms of the other vertebrates, the craniota, by secondary modifications. My thesis of the universal formation of the gastrula by folding of the blastula has now been clearly proved for all the vertebrates; so also has been Hertwig’s thesis of the origin of the middle germinal layers by the folding of a couple of cœlom-pouches which appear at the border of the primitive mouth. Just as the gastræa-theory explains the origin and identity of the two primary layers, so the cœlom-theory explains those of the four secondary layers. The point of origin is always the properistoma, the border of the original primitive mouth of the gastrula, at which the two primary layers pass directly into each other.
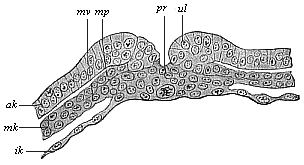
Fig. 96—Transverse section of the primitive groove (or primitive mouth) of a rabbit. (From Van Beneden.) pr primitive mouth, ul lips of same (primitive lips), ak and ik outer and inner germinal layers, mk middle germinal layer, mp parietal layer, mv visceral layer of the mesoderm.
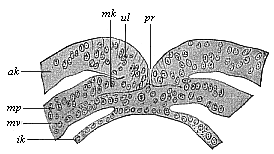
Fig. 97—Transverse section of the primitive mouth (or groove) of a human embryo (at the cœlomula stage). (From Count Spee.) pr primitive mouth, ul lips of same (primitive folds), ak and ik outer and inner germinal layers, mk middle layer, mp parietal layer, mv visceral layer of the mesoblasts.
Moreover, the cœlomula is important as the immediate source of the chordula, the embryonic reproduction of the ancient, typical, unarticulated, worm-like form, which has an axial chorda between the dorsal nerve-tube and the ventral gut-tube. This instructive chordula (Figs. 83–86) provides a valuable support of our phylogeny; it indicates the important moment in our stem-history at which the stem of the chordonia (tunicates and vertebrates) parted for ever from the divergent stems of the other metazoa (articulates, echinoderms, and molluscs).
I may express here my opinion, in the form of a chordæa-theory, that the characteristic chordula-larva of the chordonia has in reality this great significance—it is the typical reproduction (preserved by heredity) of the ancient common stem-form of all the vertebrates and tunicates, the long-extinct Chordæa. We will return in Chapter XX to these worm-like ancestors, which stand out as luminous points in the obscure stem-history of the invertebrate ancestors of our race.
We have now secured a number of firm standing-places in the labyrinthian course of our individual development by our study of the important embryonic forms which we have called the cytula, morula, blastula, gastrula, cœlomula, and chordula. But we have still in front of us the difficult task of deriving the complicated frame of the human body, with all its different parts, organs, members, etc., from the simple form of the chordula. We have previously considered the origin of this four-layered embryonic form from the two-layered gastrula. The two primary germinal layers, which form the entire body of the gastrula, and the two middle layers of the cœlomula that develop between them, are the four simple cell-strata, or epithelia, which alone go to the formation of the complex body of man and the higher animals. It is so difficult to understand this construction that we will first seek a companion who may help us out of many difficulties.
This helpful associate is the science of comparative anatomy. Its task is, by comparing the fully-developed bodily forms in the various groups of animals, to learn the general laws of organisation according to which the body is constructed; at the same time, it has to determine the affinities of the various groups by critical appreciation of the degrees of difference between them. Formerly, this work was conceived in a teleological sense, and it was sought to find traces of the plan of the Creator in the actual purposive organisation of animals. But comparative anatomy has gone much deeper since the establishment of the theory of descent; its philosophic aim now is to explain the variety of organic forms by adaptation, and their similarity by heredity. At the same time, it has to recognise in the shades of difference in form the degree of blood-relationship, and make an effort to construct the ancestral tree of the animal world. In this way, comparative anatomy enters into the closest relations with comparative embryology on the one hand, and with the science of classification on the other.
Now, when we ask what position man occupies among the other organisms according to the latest teaching of comparative anatomy and classification, and how man’s place in the zoological system is determined by comparison of the mature bodily forms, we get a very definite and significant reply; and this reply gives us extremely important conclusions that enable us to understand the embryonic development and its evolutionary purport. Since Cuvier and Baer, since the immense progress that was effected in the early decades of the nineteenth century by these two great zoologists, the opinion has generally prevailed that the whole animal kingdom may be distributed in a small number of great divisions or types. They are called types because a certain typical or characteristic structure is constantly preserved within each of these large sections. Since we applied the theory of descent to this doctrine of types, we have learned that this common type is an outcome of heredity; all the animals of one type are blood-relatives, or members of one stem, and can be traced to a common ancestral form. Cuvier and Baer set up four of these types: the vertebrates, articulates, molluscs, and radiates. The first three of these are still retained, and may be conceived as natural phylogenetic unities, as stems or phyla in the sense of the theory of descent. It is quite otherwise with the fourth type—the radiata. These animals, little known as yet at the beginning of the nineteenth century, were made to form a sort of lumber-room, into which were cast all the lower animals that did not belong to the other three types. As we obtained a closer acquaintance with them in the course of the last sixty years, it was found that we must distinguish among them from four to eight different types. In this way the total number of animal stems or phyla has been raised to eight or twelve (cf. Chapter XX).
These twelve stems of the animal kingdom are, however, by no means co-ordinate and independent types, but have definite relations, partly of subordination, to each other, and a very different phylogenetic meaning. Hence they must not be arranged simply in a row one after the other, as was generally done until thirty years ago, and is still done in some manuals. We must distribute them in three subordinate principal groups of very different value, and arrange the various stems phylogenetically on the principles which I laid down in my Monograph on the Sponges, and developed in the Study of the Gastræa Theory. We have first to distinguish the unicellular animals (protozoa) from the multicellular tissue-forming (metazoa). Only the latter exhibit the important processes of segmentation and gastrulation; and they alone have a primitive gut, and form germinal layers and tissues.
The metazoa, the tissue-animals or gut-animals, then sub-divide into two main sections, according as a body-cavity is or is not developed between the primary germinal layers. We may call these the cœlenteria and cœlomaria, the former are often also called zoophytes or cœlenterata, and the latter bilaterals. This division is the more important as the cœlenteria (without cœlom) have no blood and blood-vessels, nor an anus. The cœlomaria (with body-cavity) have generally an anus, and blood and blood-vessels. There are four stems belonging to the cœlenteria: the gastræads (“primitive-gut animals”), sponges, cnidaria, and platodes. Of the cœlomaria we can distinguish six stems: the vermalia at the bottom represent the common stem-group (derived from the platodes) of these, the other five typical stems of the cœlomaria—the molluscs, echinoderms, articulates, tunicates, and vertebrates—being evolved from them.
Man is, in his whole structure, a true vertebrate, and develops from an impregnated ovum in just the same characteristic way as the other vertebrates. There can no longer be the slightest doubt about this fundamental fact, nor of the fact that all the vertebrates form a natural phylogenetic unity, a single stem. The whole of the members of this stem, from the amphioxus and the cyclostoma to the apes and man, have the same characteristic disposition, connection, and development of the central organs, and arise in the same way from the common embryonic form of the chordula. Without going into the difficult question of the origin of this stem, we must emphasise the fact that the vertebrate stem has no direct affinity whatever to five of the other ten stems; these five isolated phyla are the sponges, cnidaria, molluscs, articulates, and echinoderms. On the other hand, there are important and, to an extent, close phylogenetic relations to the other five stems—the protozoa (through the amœbæ), the gastræads (through the blastula and gastrula), the platodes and vermalia (through the cœlomula), and the tunicates (through the chordula).
How we are to explain these phylogenetic relations in the present state of our knowledge, and what place is assigned to the vertebrates in the animal ancestral tree, will be considered later (Chapter XX). For the present our task is to make plainer the vertebrate character of man, and especially to point out the chief peculiarities of organisation by which the vertebrate stem is profoundly separated from the other eleven stems of the animal kingdom. Only after these comparative-anatomical considerations shall we be in a position to attack the difficult question of our embryology. The development of even the simplest and lowest vertebrate from the simple chordula (Figs. 83–86) is so complicated and difficult to follow that it is necessary to understand the organic features of the fully-formed vertebrate in order to grasp the course of its embryonic evolution. But it is equally necessary to confine our attention, in this general anatomic description of the vertebrate-body, to the essential facts, and pass by all the unessential. Hence, in giving now an ideal anatomic description of the chief features of the vertebrate and its internal organisation, I omit all the subordinate points, and restrict myself to the most important characteristics.
Much, of course, will seem to the reader to be essential that is only of subordinate and secondary interest, or even not essential at all, in the light of comparative anatomy and embryology. For instance, the skull and vertebral column and the extremities are non-essential in this sense. It is true that these parts are very important physiologically; but for the morphological conception of the vertebrate they are not essential, because they are only found in the higher, not the lower, vertebrates. The lowest vertebrates have neither skull nor vertebræ, and no extremities or limbs. Even the human embryo passes through a stage in which it has no skull or vertebræ; the trunk is quite simple, and there is yet no trace of arms and legs. At this stage of development man, like every other higher vertebrate, is essentially similar to the simplest vertebrate form, which we now find in only one living specimen. This one lowest vertebrate that merits the closest study—undoubtedly the most interesting of all the vertebrates after man—is the famous lancelet or amphioxus, to which we have already often referred. As we are going to study it more closely later on (Chapters XVI and XVII), I will only make one or two passing observations on it here.
The amphioxus lives buried in the sand of the sea, is about one or two inches in length, and has, when fully developed, the shape of a very simple, longish, lancet-like leaf; hence its name of the lancelet. The narrow body is compressed on both sides, almost equally pointed at the fore and hind ends, without any trace of external appendages or articulation of the body into head, neck, breast, abdomen, etc. Its whole shape is so simple that its first discoverer thought it was a naked snail. It was not until much later—half a century ago—that the tiny creature was studied more carefully, and was found to be a true vertebrate. More recent investigations have shown that it is of the greatest importance in connection with the comparative anatomy and ontogeny of the vertebrates, and therefore with human phylogeny. The amphioxus reveals the great secret of the origin of the vertebrates from the invertebrate vermalia, and in its development and structure connects directly with certain lower tunicates, the ascidia.
When we make a number of sections of the body of the amphioxus, firstly vertical longitudinal sections through the whole body from end to end, and secondly transverse sections from right to left, we get anatomic pictures of the utmost instructiveness (cf. Figs. 98–102). In the main they correspond to the ideal which we form, with the aid of comparative anatomy and ontogeny, of the primitive type or build of the vertebrate—the long-extinct form to which the whole stem owes its origin. As we take the phylogenetic unity of the vertebrate stem to be beyond dispute, and assume a common origin from a primitive stem-form for all the vertebrates, from amphioxus to man, we are justified in forming a definite morphological idea of this primitive vertebrate (Prospondylus or Vertebræa). We need only imagine a few slight and unessential changes in the real sections of the amphioxus in order to have this ideal anatomic figure or diagram of the primitive vertebrate form, as we see in Figs. 98–102. The amphioxus departs so little from this primitive form that we may, in a certain sense, describe it as a modified “primitive vertebrate.”[24]
[24] The ideal figure of the vertebrate as given in Figs. 98–102 is a hypothetical scheme or diagram, that has been chiefly constructed on the lines of the amphioxus, but with a certain attention to the comparative anatomy and ontogeny of the ascidia and appendicularia on the one hand, and of the cyclostoma and selachii on the other. This diagram has no pretension whatever to be an “exact picture,” but merely an attempt to reconstruct hypothetically the unknown and long extinct vertebrate stem-form, an ideal “archetype.”
The outer form of our hypothetical primitive vertebrate was at all events very simple, and probably more or less similar to that of the lancelet. The bilateral or bilateral-symmetrical body is stretched out lengthways and compressed at the sides (Figs. 98–100), oval in section (Figs. 101, 102). There are no external articulation and no external appendages, in the shape of limbs, legs, or fins. On the other hand, the division of the body into two sections, head and trunk, was probably clearer in Prospondylus than it is in its little-changed ancestor, the amphioxus. In both animals the fore or head-half of the body contains different organs from the trunk, and different on the dorsal from on the ventral side. As this important division is found even in the sea-squirt, the remarkable invertebrate stem-relative of the vertebrates, we may assume that it was also found in the prochordonia, the common ancestors of both stems. It is also very pronounced in the young larvæ of the cyclostoma; this fact is particularly interesting, as this palingenetic larva-form is in other respects also an important connecting-link between the higher vertebrates and the acrania.
The head of the acrania, or the anterior half of the body (both of the real amphioxus and the ideal prospondylus), contains the branchial (gill) gut and heart in the ventral section and the brain and sense-organs in the dorsal section. The trunk, or posterior half of the body, contains the hepatic (liver) gut and sexual-glands in the ventral part, and the spinal marrow and most of the muscles in the dorsal part.
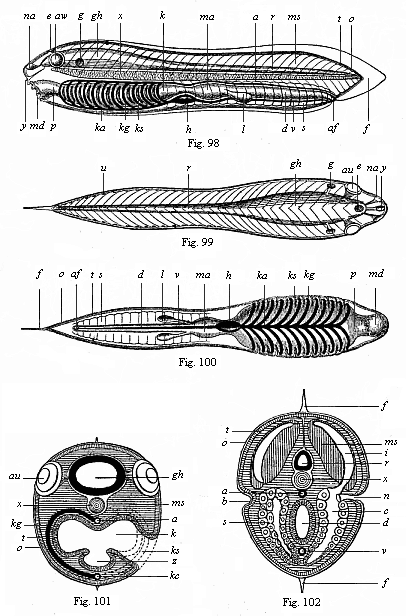
Figs. 98–102.—The ideal primitive vertebrate (prospondylus). Diagram. Fig. 98 side-view (from the left). Fig. 99 back-view. Fig. 100 front view. Fig. 101 transverse section through the head (to the left through the gill-pouches, to the right through the gill-clefts). Fig. 102 transverse section of the trunk (to the right a pro-renal canal is affected). a aorta, af anus, au eye, b lateral furrow (primitive renal process), c cœloma (body-cavity), d small intestine, e parietal eye (epiphysis), f fin border of the skin, g auditory vesicle, gh brain, h heart, i muscular cavity (dorsal cœlom-pouch), k gill-gut, ka gill-artery, kg gill-arch, ks gill-folds, l liver, ma stomach, md mouth, ms muscles, na nose (smell pit), n renal canals, u apertures of same, o outer skin, p gullet, r spinal marrow, a sexual glands (gonads), t corium, u kidney-openings (pores of the lateral furrow), v visceral vein (chief vein). x chorda, y hypophysis (urinary appendage), z gullet-groove or gill-groove (hypobranchial groove).
In the longitudinal section of the ideal vertebrate (Fig. 98) we have in the middle of the body a thin and flexible, but stiff, cylindrical rod, pointed at both ends (ch). It goes the whole length through the middle of the body, and forms, as the central skeletal axis, the original structure of the later vertebral column. This is the axial rod, or chorda dorsalis, also called chorda vertebralis, vertebral cord, axial cord, dorsal cord, notochorda, or, briefly, chorda. This solid, but flexible and elastic, axial rod consists of a cartilaginous mass of cells, and forms the inner axial skeleton or central frame of the body; it is only found in vertebrates and tunicates, not in any other animals. As the first structure of the spinal column it has the same radical significance in all vertebrates, from the amphioxus to man. But it is only in the amphioxus and the cyclostoma that the axial rod retains its simplest form throughout life. In man and all the higher vertebrates it is found only in the earlier embryonic period, and is afterwards replaced by the articulated vertebral column.
The axial rod or chorda is the real solid chief axis of the vertebrate body, and at the same time corresponds to the ideal long-axis, and serves to direct us with some confidence in the orientation of the principal organs. We therefore take the vertebrate-body in its original, natural disposition, in which the long-axis lies horizontally, the dorsal side upward and the ventral side downward (Fig. 98). When we make a vertical section through the whole length of this long axis, the body divides into two equal and symmetrical halves, right and left. In each half we have originally the same organs in the same disposition and connection; only their disposal in relation to the vertical plane of section, or median plane, is exactly reversed: the left half is the reflection of the right. We call the two halves antimera (opposed-parts). In the vertical plane of section that divides the two halves the sagittal (“arrow”) axis, or “dorsoventral axis,” goes from the back to the belly, corresponding to the sagittal seam of the skull. But when we make a horizontal longitudinal section through the chorda, the whole body divides into a dorsal and a ventral half. The line of section that passes through the body from right to left is the transverse, frontal, or lateral axis.
The two halves of the vertebrate body that are separated by this horizontal transverse axis and by the chorda have quite different characters. The dorsal half is mainly the animal part of the body, and contains the greater part of what are called the animal organs, the nervous system, muscular system, osseous system, etc.—the instruments of movement and sensation. The ventral half is essentially the vegetative half of the body, and contains the greater part of the vertebrate’s vegetal organs, the visceral and vascular systems, sexual system, etc.—the instruments of nutrition and reproduction. Hence in the construction of the dorsal half it is chiefly the outer, and in the construction of the ventral half chiefly the inner, germinal layer that is engaged. Each of the two halves develops in the shape of a tube, and encloses a cavity in which another tube is found. The dorsal half contains the narrow spinal-column cavity or vertebral canal above the chorda, in which lies the tube-shaped central nervous system, the medullary tube. The ventral half contains the much more spacious visceral cavity or body-cavity underneath the chorda, in which we find the alimentary canal and all its appendages.
The medullary tube, as the central nervous system or psychic organ of the vertebrate is called in its first stage, consists, in man and all the higher vertebrates, of two different parts: the large brain, contained in the skull, and the long spinal cord which stretches from there over the whole dorsal part of the trunk. Even in the primitive vertebrate this composition is plainly indicated. The fore half of the body, which corresponds to the head, encloses a knob-shaped vesicle, the brain (gh); this is prolonged backwards into the thin cylindrical tube of the spinal marrow (r). Hence we find here this very important psychic organ, which accomplishes sensation, will, and thought, in the vertebrates, in its simplest form. The thick wall of the nerve-tube, which runs through the long axis of the body immediately over the axial rod, encloses a narrow central canal filled with fluid (Figs. 98–102 r). We still find the medullary tube in this very simple form for a time in the embryo of all the vertebrates, and it retains this form in the amphioxus throughout life; only in the latter case the cylindrical medullary tube barely indicates the separation of brain and spinal cord. The lancelet’s medullary tube runs nearly the whole length of the body, above the chorda, in the shape of a long thin tube of almost equal diameter throughout, and there is only a slight swelling of it right at the front to represent the rudiment of a cerebral lobe. It is probable that this peculiarity of the amphioxus is connected with the partial atrophy of its head, as the ascidian larvæ on the one hand and the young cyclostoma on the other clearly show a division of the vesicular brain, or head marrow, from the thinner, tubular spinal marrow.
Probably we must trace to the same phylogenetic cause the defective nature of the sense organs of the amphioxus, which we will describe later (Chapter XVI). Prospondylus, on the other hand, probably had three pairs of sense-organs, though of a simple character, a pair of, or a single olfactory depression, right in front (Figs. 98, 99, na), a pair of eyes (au) in the lateral walls of the brain, and a pair of simple auscultory vesicles (g) behind. There was also, perhaps, a single parietal or “pineal” eye at the top of the skull (epiphysis, e).
In the vertical median plane (or middle plane, dividing the bilateral body into right and left halves) we have in the acrania, underneath the chorda, the mesentery and visceral tube, and above it the medullary tube; and above the latter a membranous partition of the two halves of the body. With this partition is connected the mass of connective tissue which acts as a sheath both for the medullary tube and the underlying chorda, and is, therefore, called the chord-sheath (perichorda); it originates from the dorsal and median part of the cœlom-pouches, which we shall call the skeleton plate or “sclerotom” in the craniote embryo. In the latter the chief part of the skeleton—the vertebral column and skull—develops from this chord-sheath; in the acrania it retains its simple form as a soft connective matter, from which are formed the membranous partitions between the various muscular plates or myotomes (Figs. 98, 99 ms).
To the right and left of the cord-sheath, at each side of the medullary tube and the underlying axial rod, we find in all the vertebrates the large masses of muscle that constitute the musculature of the trunk and effect its movements. Although these are very elaborately differentiated and connected in the developed vertebrate (corresponding to the various parts of the bony skeleton), in our ideal primitive vertebrate we can distinguish only two pairs of these principal muscles, which run the whole length of the body parallel to the chorda. These are the upper (dorsal) and lower (ventral) lateral muscles of the trunk. The upper (dorsal) muscles, or the original dorsal muscles (Fig. 102 ms), form the thick mass of flesh on the back. The lower (ventral) muscles, or the original muscles of the belly, form the fleshy wall of the abdomen. Both sets are segmented, and consist of a double row of muscular plates (Figs. 98, 99 ms); the number of these myotomes determines the number of joints in the trunk, or metamera. The myotomes are also developed from the thick wall of the cœlom-pouches (Fig. 102 i).
Outside this muscular tube we have the external envelope of the vertebrate body, which is known as the corium or cutis. This strong and thick envelope consists, in its deeper strata, chiefly of fat and loose connective tissue, and in its upper layers of cutaneous muscles and firmer connective tissue. It covers the whole surface of the fleshy body, and is of considerable thickness in all the craniota. But in the acrania the corium is merely a thin plate of connective tissue, an insignificant “corium-plate” (lamella corii, Figs. 98–102 t).
Immediately above the corium is the outer skin (epidermis, o), the general covering of the whole outer surface. In the higher vertebrates the hairs, nails, feathers, claws, scales, etc., grow out of this epidermis. It consists, with all its appendages and products, of simple cells, and has no blood-vessels. Its cells are connected with the terminations of the sensory nerves. Originally, the outer skin is a perfectly simple covering of the outer surface of the body, composed only of homogeneous cells—a permanent horn-plate. In this simplest form, as a one-layered epithelium, we find it, at first, in all the vertebrates, and throughout life in the acrania. It afterwards grows thicker in the higher vertebrates, and divides into two strata—an outer, firmer corneous (horn) layer and an inner, softer mucus-layer; also a number of external and internal appendages grow out of it: outwardly, the hairs, nails, claws, etc., and inwardly, the sweat-glands, fat-glands, etc.
It is probable that in our primitive vertebrate the skin was raised in the middle line of the body in the shape of a vertical fin border (f). A similar fringe, going round the greater part of the body, is found to-day in the amphioxus and the cyclostoma; we also find one in the tail of fish-larvæ and tadpoles.
Now that we have considered the external parts of the vertebrate and the animal organs, which mainly lie in the dorsal half, above the chorda, we turn to the vegetal organs, which lie for the most part in the ventral half, below the axial rod. Here we find a large body-cavity or visceral cavity in all the craniota. The spacious cavity that encloses the greater part of the viscera corresponds to only a part of the original cœloma, which we considered in Chapter X; hence it nay be called the metacœloma. As a rule, it is still briefly called the cœloma; formerly it was known in anatomy as the pleuroperitoneal cavity. In man and the other mammals (but only in these) this cœloma divides, when fully developed, into two different cavities, which are separated by a transverse partition—the muscular diaphragm. The fore or pectoral cavity (pleura-cavity) contains the œsophagus (gullet), heart, and lungs; the hind or peritoneal or abdominal cavity contains the stomach, small and large intestines, liver, pancreas, kidneys, etc. But in the vertebrate embryo, before the diaphragm is developed, the two cavities form a single continuous body-cavity, and we find it thus in all the lower vertebrates throughout life. This body-cavity is clothed with a delicate layer of cells, the cœlom-epithelium. In the acrania the cœlom is segmented both dorsally and ventrally, as their muscular pouches and primitive genital organs plainly show (Fig. 102).
The chief of the viscera in the body-cavity is the alimentary canal, the organ that represents the whole body in the gastrula. In all the vertebrates it is a long tube, enclosed in the body-cavity and more or less differentiated in length, and has two apertures—a mouth for taking in food (Figs. 98, 100 md) and an anus for the ejection of unusable matter or excrements (af). With the alimentary canal a number of glands are connected which are of great importance for the vertebrate body, and which all grow out of the canal. Glands of this kind are the salivary glands, the lungs, the liver, and many smaller glands. Nearly all these glands are wanting in the acrania; probably there were merely a couple of simple hepatic tubes (Figs. 98, 100 l) in the vertebrate stem-form. The wall of the alimentary canal and all its appendages consists of two different layers; the inner, cellular clothing is the gut-gland-layer, and the outer, fibrous envelope consists of the gut-fibre-layer; it is mainly composed of muscular fibres which accomplish the digestive movements of the canal, and of connective-tissue fibres that form a firm envelope. We have a continuation of it in the mesentery, a thin, bandage-like layer, by means of which the alimentary canal is fastened to the ventral side of the chorda, originally the dorsal partition of the two cœlom-pouches. The alimentary canal is variously modified in the vertebrates both as a whole and in its several sections, though the original structure is always the same, and is very simple. As a rule, it is longer (often several times longer) than the body, and therefore folded and winding within the body-cavity, especially at the lower end. In man and the higher vertebrates it is divided into several sections, often separated by valves—the mouth, pharynx, œsophagus, stomach, small and large intestine, and rectum. All these parts develop from a very simple structure, which originally (throughout life in the amphioxus) runs from end to end under the chorda in the shape of a straight cylindrical canal.
As the alimentary canal may be regarded morphologically as the oldest and most important organ in the body, it is interesting to understand its essential features in the vertebrate more fully, and distinguish them from unessential features. In this connection we must particularly note that the alimentary canal of every vertebrate shows a very characteristic division into two sections—a fore and a hind chamber. The fore chamber is the head-gut or branchial gut (Figs. 98–100 p, k), and is chiefly occupied with respiration. The hind section is the trunk-gut or hepatic gut, which accomplishes digestion (ma, d). In all vertebrates there are formed, at an early stage, to the right and left in the fore-part of the head-gut, certain special clefts that have an intimate connection with the original respiratory apparatus of the vertebrate—the branchial (gill) clefts (ks). All the lower vertebrates, the lancelets, lampreys, and fishes, are constantly taking in water at the mouth, and letting it out again by the lateral clefts of the gullet. This water serves for breathing. The oxygen contained in it is inspired by the blood-canals, which spread out on the parts between the gill-clefts, the gill-arches (kg). These very characteristic branchial clefts and arches are found in the embryo of man and all the higher vertebrates at an early stage of development, just as we find them throughout life in the lower vertebrates. However, these clefts and arches never act as respiratory organs in the mammals, birds, and reptiles, but gradually develop into quite different parts. Still, the fact that they are found at first in the same form as in the fishes is one of the most interesting proofs of the descent of these three higher classes from the fishes.
Not less interesting and important is an organ that develops from the ventral wall in all vertebrates—the gill-groove or hypobranchial groove. In the acrania and the ascidiæ it consists throughout life of a glandular ciliated groove, which runs down from the mouth in the ventral middle line of the gill-gut, and takes small particles of food to the stomach (Fig. 101 z). But in the craniota the thyroid gland (thyreoidea) is developed from it, the gland that lies in front of the larynx, and which, when pathologically enlarged, forms goitre (struma).
From the head-gut we get not only the gills, the organs of water-breathing in the lower vertebrates, but also the lungs, the organs of atmospheric breathing in the five higher classes. In these cases a vesicular fold appears in the gullet of the embryo at an early stage, and gradually takes the shape of two spacious sacs, which are afterwards filled with air. These sacs are the two air-breathing lungs, which take the place of the water-breathing gills. But the vesicular invagination, from which the lungs arise, is merely the familiar air-filled vesicle, which we call the floating-bladder of the fish, and which alters its specific weight, acting as hydrostatic organ or floating apparatus. This structure is not found in the lowest vertebrate classes—the acrania and cyclostoma. We shall see more of it in Volume II.
The second chief section of the vertebrate-gut, the trunk or liver-gut, which accomplishes digestion, is of very simple construction in the acrania. It consists of two different chambers. The first chamber, immediately behind the gill-gut, is the expanded stomach (ma); the second, narrower and longer chamber, is the straight small intestine (d): it issues behind on the ventral side by the anus (af). Near the limit of the two chambers in the visceral cavity we find the liver, in the shape of a simple tube or blind sac (l); in the amphioxus it is single; in the prospondylus it was probably double (Figs. 98, 100 l).
Closely related morphologically and physiologically to the alimentary canal is the vascular system of the vertebrate, the chief sections of which develop from the fibrous gut-layer. It consists of two different but directly connected parts, the system of blood-vessels and that of lymph-vessels. In the passages of the one we find red blood, and in the other colourless lymph. To the lymphatic system belong, first of all, the lymphatic canals proper or absorbent veins, which are distributed among all the organs, and absorb the used-up juices from the tissues, and conduct them into the venous blood; but besides these there are the chyle-vessels, which absorb the white chyle, the milky fluid prepared by the alimentary canal from the food, and conduct this also to the blood.
The blood-vessel system of the vertebrate has a very elaborate construction, but seems to have had a very simple form in the primitive vertebrate, as we find it to-day permanently in the annelids (for instance, earth-worms) and the amphioxus. We accordingly distinguish first of all as essential, original parts of it two large single blood-canals, which lie in the fibrous wall of the gut, and run along the alimentary canal in the median plane of the body, one above and the other underneath the canal. These principal canals give out numerous branches to all parts of the body, and pass into each other by arches before and behind; we will call them the primitive artery and the primitive vein. The first corresponds to the dorsal vessel, the second to the ventral vessel, of the worms. The primitive or principal artery, usually called the aorta (Fig. 98 a), lies above the gut in the middle line of its dorsal side, and conducts oxidised or arterial blood from the gills to the body. The primitive or principal vein (Fig. 100 v) lies below the gut, in the middle line of its ventral side, and is therefore also called the vena subintestinalis; it conducts carbonised or venous blood back from the body to the gills. At the branchial section of the gut in front the two canals are connected by a number of branches, which rise in arches between the gill-clefts. These “branchial vascular arches” (kg) run along the gill-arches, and have a direct share in the work of respiration. The anterior continuation of the principal vein which runs on the ventral wall of the gill-gut, and gives off these vascular arches upwards, is the branchial artery (ka). At the border of the two sections of the ventral vessel it enlarges into a contractile spindle-shaped tube (Figs. 98, 100 h). This is the first outline of the heart, which afterwards becomes a four-chambered pump in the higher vertebrates and man. There is no heart in the amphioxus, probably owing to degeneration. In prospondylus the ventral gill-heart probably had the simple form in which we still find it in the ascidia and the embryos of the craniota (Figs. 98, 100 h).
The kidneys, which act as organs of excretion or urinary organs in all vertebrates, have a very different and elaborate construction in the various sections of this stem; we will consider them further in Chapter 2.29. Here I need only mention that in our hypothetical primitive vertebrate they probably had the same form as in the actual amphioxus—the primitive kidneys (protonephra). These are originally made up of a double row of little canals, which directly convey the used-up juices or the urine out of the body-cavity (Fig. 102 n). The inner aperture of these pronephridial canals opens with a ciliated funnel into the body-cavity; the external aperture opens in lateral grooves of the epidermis, a couple of longitudinal grooves in the lateral surface of the outer skin (Fig. 102 b). The pronephridial duct is formed by the closing of this groove to the right and left at the sides. In all the craniota it develops at an early stage in the horny plate; in the amphioxus it seems to be converted into a wide cavity, the atrium, or peribranchial space.
Next to the kidneys we have the sexual organs of the vertebrate. In most of the members of this stem the two are united in a single urogenital system; it is only in a few groups that the urinary and sexual organs are separated (in the amphioxus, the cyclostoma, and some sections of the fish-class). In man and all the higher vertebrates the sexual apparatus is made up of various parts, which we will consider in Chapter XXIX. But in the two lowest classes of our stem, the acrania and cyclostoma, they consist merely of simple sexual glands or gonads, the ovaries of the female sex and the testicles (spermaria) of the male; the former provide the ova, the latter the sperm. In the craniota we always find only one pair of gonads; in the amphioxus several pairs, arranged in succession. They must have had the same form in our hypothetical prospondylus (Figs. 98, 100 s). These segmental pairs of gonads are the original ventral halves of the cœlom-pouches.
The organs which we have now enumerated in this general survey, and of which we have noted the characteristic disposition, are those parts of the organism that are found in all vertebrates without exception in the same relation to each other, however much they may be modified. We have chiefly had in view the transverse section of the body (Figs. 101, 102), because in this we see most clearly the distinctive arrangement of them. But to complete our picture we must also consider the segmentation or metamera-formation of them, which has yet been hardly noticed, and which is seen best in the longitudinal section. In man and all the more advanced vertebrates the body is made up of a series or chain of similar members, which succeed each other in the long axis of the body—the segments or metamera of the organism. In man these homogeneous parts number thirty-three in the trunk, but they run to several hundred in many of the vertebrates (such as serpents or eels). As this internal articulation or metamerism is mainly found in the vertebral column and the surrounding muscles, the sections or metamera were formerly called pro-vertebræ. As a fact, the articulation is by no means chiefly determined and caused by the skeleton, but by the muscular system and the segmental arrangement of the kidneys and gonads. However, the composition from these pro-vertebræ or internal metamera is usually, and rightly, put forward as a prominent character of the vertebrate, and the manifold division or differentiation of them is of great importance in the various groups of the vertebrates. But as far as our present task—the derivation of the simple body of the primitive vertebrate from the chordula—is concerned, the articulate parts or metamera are of secondary interest, and we need not go into them just now.
Fig. 103 A, B, C, D.—Instances of redundant mammary glands and nipples (hypermastism). A a pair of small redundant breasts (with two nipples on the left) above the large normal ones; from a 45-year-old Berlin woman, who had had children 17 times (twins twice). (From Hansemann.) B the highest number: ten nipples (all giving milk), three pairs above, one pair below, the large normal breasts; from a 22-year-old servant at Warschau. (From Neugebaur.) C three pairs of nipples: two pairs on the normal glands and one pair above; from a 19-year-old Japanese girl. D four pairs of nipples: one pair above the normal and two pairs of small accessory nipples underneath; from a 22-year-old Bavarian soldier. (From Wiedersheim.)
The characteristic composition of the vertebrate body develops from the embryonic structure in the same way in man as in all the other vertebrates. As all competent experts now admit the monophyletic origin of the vertebrates on the strength of this significant agreement, and this “common descent of all the vertebrates from one original stem-form” is admitted as an historical fact, we have found the answer to “the question of questions.” We may, moreover, point out that this answer is just as certain and precise in the case of the origin of man from the mammals. This advanced vertebrate class is also monophyletic, or has evolved from one common stem-group of lower vertebrates (reptiles, and, earlier still, amphibia). This follows from the fact that the mammals are clearly distinguished from the other classes of the stem, not merely in one striking particular, but in a whole group of distinctive characters.
It is only in the mammals that we find the skin covered with hair, the breast-cavity separated from the abdominal cavity by a complete diaphragm, and the larynx provided with an epiglottis. The mammals alone have three small auscultory bones in the tympanic cavity—a feature that is connected with the characteristic modification of their maxillary joint. Their red blood-cells have no nucleus, whereas this is retained in all other vertebrates. Finally, it is only in the mammals that we find the remarkable function of the breast structure which has given its name to the whole class—the feeding of the young by the mother’s milk. The mammary glands which serve this purpose are interesting in so many ways that we may devote a few lines to them here.
As is well known, the lower mammals, especially those which beget a number of young at a time, have several mammary glands at the breast. Hedgehogs and sows have five pairs, mice four or five pairs, dogs and squirrels four pairs, cats and bears three pairs, most of the ruminants and many of the rodents two pairs, each provided with a teat or nipple (mastos). In the various genera of the half-apes (lemurs) the number varies a good deal. On the other hand, the bats and apes, which only beget one young at a time as a rule, have only one pair of mammary glands, and these are found at the breast, as in man.
These variations in the number or structure of the mammary apparatus (mammarium) have become doubly interesting in the light of recent research in comparative anatomy. It has been shown that in man and the apes we often find redundant mammary glands (hyper-mastism) and corresponding teats (hyper-thelism) in both sexes. Fig. 103 shows four cases of this kind—A, B, and C of three women, and D of a man. They prove that all the above-mentioned numbers may be found occasionally in man. Fig. 103 A shows the breast of a Berlin woman who had had children seventeen times, and who has a pair of small accessory breasts (with two nipples on the left one) above the two normal breasts; this is a common occurrence, and the small soft pad above the breast is not infrequently represented in ancient statues of Venus. In Fig. 103 C we have the same phenomenon in a Japanese girl of nineteen, who has two nipples on each breast besides (three pairs altogether). Fig. 103 D is a man of twenty-two with four pairs of nipples (as in the dog), a small pair above and two small pairs beneath the large normal teats. The maximum number of five pairs (as in the sow and hedgehog) was found in a Polish servant of twenty-two who had had several children; milk was given by each nipple; there were three pairs of redundant nipples above and one pair underneath the normal and very large breasts (Fig. 103 B).
A number of recent investigations (especially among recruits) have shown that these things are not uncommon in the male as well as the female sex. They can only be explained by evolution, which attributes them to atavism and latent heredity. The earlier ancestors of all the primates (including man) were lower placentals, which had, like the hedgehog (one of the oldest forms of the living placentals), several mammary glands (five or more pairs) in the abdominal skin. In the apes and man only a couple of them are normally developed, but from time to time we get a development of the atrophied structures. Special notice should be taken of the arrangement of these accessory mammæ; they form, as is clearly seen in Fig. 103 B and D, two long rows, which diverge forward (towards the arm-pit), and converge behind in the middle line (towards the loins). The milk-glands of the polymastic lower placentals are arranged in similar lines.
The phylogenetic explanation of polymastism, as given in comparative anatomy, has lately found considerable support in ontogeny. Hans Strahl, E. Schmitt, and others, have found that there are always in the human embryo at the sixth week (when it is three-fifths of an inch long) the microscopic traces of five pairs of mammary glands, and that they are arranged at regular distances in two lateral and divergent lines, which correspond to the mammary lines. Only one pair of them—the central pair—are normally developed, the others atrophying. Hence there is for a time in the human embryo a normal hyperthelism, and this can only be explained by the descent of man from lower primates (lemurs) with several pairs.
But the milk-gland of the mammal has a great morphological interest from another point of view. This organ for feeding the young in man and the higher mammals is, as is known, found in both sexes. However, it is usually active only in the female sex, and yields the valuable “mother’s milk”; in the male sex it is small and inactive, a real rudimentary organ of no physiological interest. Nevertheless, in certain cases we find the breast as fully developed in man as in woman, and it may give milk for feeding the young.
We have a striking instance of this gynecomastism (large milk-giving breasts in a male) in Fig. 104. I owe the photograph (taken from life) to the kindness of Dr. Ornstein, of Athens, a German physician, who has rendered service by a number of anthropological observations, (for instance, in several cases of tailed men). The gynecomast in question is a Greek recruit in his twentieth year, who has both normally developed male organs and very pronounced female breasts. It is noteworthy that the other features of his structure are in accord with the softer forms of the female sex. It reminds us of the marble statues of hermaphrodites which the ancient Greek and Roman sculptors often produced. But the man would only be a real hermaphrodite if he had ovaries internally besides the (externally visible) testicles.
I observed a very similar case during my stay in Ceylon (at Belligemma) in 1881. A young Cinghalese in his twenty-fifth year was brought to me as a curious hermaphrodite, half-man and half-woman. His large breasts gave plenty of milk; he was employed as “male nurse” to suckle a new-born infant whose mother had died at birth. The outline of his body was softer and more feminine than in the Greek shown in Fig. 104. As the Cinghalese are small of stature and of graceful build, and as the men often resemble the women in clothing (upper part of the body naked, female dress on the lower part) and the dressing of the hair (with a comb), I first took the beardless youth to be a woman. The illusion was greater, as in this remarkable case gynecomastism was associated with cryptorchism—that is to say, the testicles had kept to their original place in the visceral cavity, and had not travelled in the normal way down into the scrotum. (Cf. Chapter XXIX.) Hence the latter was very small, soft, and empty. Moreover, one could feel nothing of the testicles in the inguinal canal. On the other hand, the male organ was very small, but normally developed. It was clear that this apparent hermaphrodite also was a real male.
Another case of practical gynecomastism has been described by Alexander von Humboldt. In a South American forest he found a solitary settler whose wife had died in child-birth. The man had laid the new-born child on his own breast in despair; and the continuous stimulus of the child’s sucking movements had revived the activity of the mammary glands. It is possible that nervous suggestion had some share in it. Similar cases have been often observed in recent years, even among other male mammals (such as sheep and goats).
The great scientific interest of these facts is in their bearing on the question of heredity. The stem-history of the mammarium rests partly on its embryology (Chapter XXIV.) and partly on the facts of comparative anatomy and physiology. As in the lower and higher mammals (the monotremes, and most of the marsupials) the whole lactiferous apparatus is only found in the female; and as there are traces of it in the male only in a few younger marsupials, there can be no doubt that these important organs were originally found only in the female mammal, and that they were acquired by these through a special adaptation to habits of life.
Later, these female organs were communicated to both sexes by heredity; and they have been maintained in all persons of either sex, although they are not physiologically active in the males. This normal permanence of the female lactiferous organs in both sexes of the higher mammals and man is independent of any selection, and is a fine instance of the much-disputed “inheritance of acquired characters.”
The three higher classes of vertebrates which we call the amniotes—the mammals, birds, and reptiles—are notably distinguished by a number of peculiarities of their development from the five lower classes of the stem—the animals without an amnion (the anamnia). All the amniotes have a distinctive embryonic membrane known as the amnion (or “water-membrane”), and a special embryonic appendage—the allantois. They have, further, a large yelk-sac, which is filled with food-yelk in the reptiles and birds, and with a corresponding clear fluid in the mammals. In consequence of these later-acquired structures, the original features of the development of the amniotes are so much altered that it is very difficult to reduce them to the palingenetic embryonic processes of the lower amnion-less vertebrates. The gastræa theory shows us how to do this, by representing the embryology of the lowest vertebrate, the skull-less amphioxus, as the original form, and deducing from it, through a series of gradual modifications, the gastrulation and cœlomation of the craniota.
It was somewhat fatal to the true conception of the chief embryonic processes of the vertebrate that all the older embryologists, from Malpighi (1687) and Wolff (1750) to Baer (1828) and Remak (1850), always started from the investigation of the hen’s egg, and transferred to man and the other vertebrates the impressions they gathered from this. This classical object of embryological research is, as we have seen, a source of dangerous errors. The large round food-yelk of the bird’s egg causes, in the first place, a flat discoid expansion of the small gastrula, and then so distinctive a development of this thin round embryonic disk that the controversy as to its significance occupies a large part of embryological literature.
One of the most unfortunate errors that this led to was the idea of an original antithesis of germ and yelk. The latter was regarded as a foreign body, extrinsic to the real germ, whereas it is properly a part of it, an embryonic organ of nutrition. Many authors said there was no trace of the embryo until a later stage, and outside the yelk; sometimes the two-layered embryonic disk itself, at other times only the central portion of it (as distinguished from the germinative area, which we will describe presently), was taken to be the first outline of the embryo. In the light of the gastræa theory it is hardly necessary to dwell on the defects of this earlier view and the erroneous conclusions drawn from it. In reality, the first segmentation-cell, and even the stem-cell itself and all that issues therefrom, belong to the embryo. As the large original yelk-mass in the undivided egg of the bird only represents an inclosure in the greatly enlarged ovum, so the later contents of its embryonic yelk-sac (whether yet segmented or not) are only a part of the entoderm which forms the primitive gut. This is clearly shown by the ova of the amphibia and cyclostoma, which explain the transition from the yelk-less ova of the amphioxus to the large yelk-filled ova of the reptiles and birds.
Fig. 105—Severance of the discoid mammal embryo from the yelk-sac, in transverse section (diagrammatic). A The germinal disk (h, hf) lies flat on one side of the branchial-gut vesicle (kb). B In the middle of the germinal disk we find the medullary groove (mr), and underneath it the chorda (ch). C The gut-fibre-layer (df) has been enclosed by the gut-gland-layer (dd). D The skin-fibre-layer (hf) and gut-fibre-layer (df) divide at the periphery; the gut (d) begins to separate from the yelk-sac or umbilical vesicle (nb). E The medullary tube (mr) is closed; the body-cavity (c) begins to form. F The provertebræ (w) begin to grow round the medullary tube (mr) and the chorda (ch): the gut (d) is cut off from the umbilical vesicle (nb). H The vertebræ (w) have grown round the medullary tube (mr) and chorda; the body-cavity is closed, and the umbilical vesicle has disappeared. The amnion and serous membrane are omitted. The letters have the same meaning throughout: h horn-plate, mr medullary tube, hf skin-fibre-layer, w provertebræ, ch chorda, c body-cavity or cœloma, df gut-fibre-layer, dd gut-gland-layer, d gut-cavity, nb umbilical vesicle.
It is precisely in the study of these difficult features that we see the incalculable value of phylogenetic considerations in explaining complex ontogenetic facts, and the need of separating cenogenetic phenomena from palingenetic. This is particularly clear as regards the comparative embryology of the vertebrates, because here the phylogenetic unity of the stem has been already established by the well-known facts of paleontology and comparative anatomy. If this unity of the stem, on the basis of the amphioxus, were always borne in mind, we should not have these errors constantly recurring.
In many cases the cenogenetic relation of the embryo to the food-yelk has until now given rise to a quite wrong idea of the first and most important embryonic processes in the higher vertebrates, and has occasioned a number of false theories in connection with them. Until thirty years ago the embryology of the higher vertebrates always started from the position that the first structure of the embryo is a flat, leaf-shaped disk; it was for this reason that the cell-layers that compose this germinal disk (also called germinative area) are called “germinal layers.” This flat germinal disk, which is round at first and then oval, and which is often described as the tread or cicatricula in the laid hen’s egg, is found at a certain part of the surface of the large globular food-yelk. I am convinced that it is nothing else than the discoid, flattened gastrula of the birds. At the beginning of germination the flat embryonic disk curves outwards, and separates on the inner side from the underlying large yelk-ball. In this way the flat layers are converted into tubes, their edges folding and joining together (Fig. 105). As the embryo grows at the expense of the food-yelk, the latter becomes smaller and smaller; it is completely surrounded by the germinal layers. Later still, the remainder of the food-yelk only forms a small round sac, the yelk-sac or umbilical vesicle (Fig. 105 nb). This is enclosed by the visceral layer, is connected by a thin stalk, the yelk-duct, with the central part of the gut-tube, and is finally, in most of the vertebrates, entirely absorbed by this (H). The point at which this takes place, and where the gut finally closes, is the visceral navel. In the mammals, in which the remainder of the yelk-sac remains without and atrophies, the yelk-duct at length penetrates the outer ventral wall. At birth the umbilical cord proceeds from here, and the point of closure remains throughout life in the skin as the navel.
As the older embryology of the higher vertebrates was mainly based on the chick, and regarded the antithesis of embryo (or formative-yelk) and food-yelk (or yelk-sac) as original, it had also to look upon the flat leaf-shaped structure of the germinal disk as the primitive embryonic form, and emphasise the fact that hollow grooves were formed of these flat layers by folding, and closed tubes by the joining together of their edges.
This idea, which dominated the whole treatment of the embryology of the higher vertebrates until thirty years ago, was totally false. The gastræa theory, which has its chief application here, teaches us that it is the very reverse of the truth. The cup-shaped gastrula, in the body-wall of which the two primary germinal layers appear from the first as closed tubes, is the original embryonic form of all the vertebrates, and all the multicellular invertebrates; and the flat germinal disk with its superficially expanded germinal layers is a later, secondary form, due to the cenogenetic formation of the large food-yelk and the gradual spread of the germ-layers over its surface. Hence the actual folding of the germinal layers and their conversion into tubes is not an original and primary, but a much later and tertiary, evolutionary process. In the phylogeny of the vertebrate embryonic process we may distinguish the following three stages:—
| A. First stage: Primary (palingenic) embryonic process. | B.
Second stage: Secondary (cenogenetic) embryonic process. | C. Third stage: Tertiary (cenogenetic) embryonic process. |
| The germinal layers form from the first closed
tubes, the one-layered blastula being converted into the two-layered gastrula
by invagination. No food-yelk. (Amphioxus.) | The germinal layers spread out leaf-wise, food-yelk gathering in
the ventral entoderm, and a large yelk-sac being formed from the middle of the
gut-tube. (Amphibia.) | The germinal layers form a flat germinal disk, the
borders of which join together and form closed tubes, separating from the
central yelk-sac. (Amniotes.) |
As this theory, a logical conclusion from the gastræa theory, has been fully substantiated by the comparative study of gastrulation in the last few decades, we must exactly reverse the hitherto prevalent mode of treatment. The yelk-sac is not to be treated, as was done formerly, as if it were originally antithetic to the embryo, but as an essential part of it, a part of its visceral tube. The primitive gut of the gastrula has, on this view, been divided into two parts in the higher animals as a result of the cenogenetic formation of the food-yelk—the permanent gut (metagaster), or permanent alimentary canal, and the yelk-sac (lecithoma), or umbilical vesicle. This is very clearly shown by the comparative ontogeny of the fishes and amphibia. In these cases the whole yelk undergoes cleavage at first, and forms a yelk-gland, composed of yelk-cells, in the ventral wall of the primitive gut. But it afterwards becomes so large that a part of the yelk does not divide, and is used up in the yelk-sac that is cut off outside.
When we make a comparative study of the embryology of the amphioxus, the frog, the chick, and the rabbit, there cannot, in my opinion, be any further doubt as to the truth of this position, which I have held for thirty years. Hence in the light of the gastræa theory we must regard the features of the amphioxus as the only and real primitive structure among all the vertebrates, departing very little from the palingenetic embryonic form. In the cyclostoma and the frog these features are, on the whole, not much altered cenogenetically, but they are very much so in the chick, and most of all in the rabbit. In the bell-gastrula of the amphioxus and in the hooded gastrula of the lamprey and the frog the germinal layers are found to be closed tubes or vesicles from the first. On the other hand, the chick-embryo (in the new laid, but not yet hatched, egg) is a flat circular disk, and it was not easy to recognise this as a real gastrula. Rauber and Goette have, however, achieved this. As the discoid gastrula grows round the large globular yelk, and the permanent gut then separates from the outlying yelk-sac, we find all the processes which we have shown (diagrammatically) in Figure 1.108—processes that were hitherto regarded as principal acts, whereas they are merely secondary.
Fig. 106—The
visceral embryonic vesicle (blastocystis or gastrocystis) of
a rabbit (the “blastula” or vesicula blastodermica of other
writers), a outer envelope (ovolemma), b skin-layer or ectoderm,
forming the entire wall of the yelk-vesicle, c groups of dark cells,
representing the visceral layer or entoderm.
Fig. 107—The
same in section. Letters as above. d cavity of the vesicle. (From
Bischoff.)
The oldest, oviparous mammals, the monotremes, behave in the same way as the reptiles and birds. But the corresponding embryonic processes in the viviparous mammals, the marsupials and placentals, are very elaborate and distinctive. They were formerly quite misinterpreted; it was not until the publication of the studies of Edward van Beneden (1875) and the later research of Selenka, Kuppfer, Rabl, and others, that light was thrown on them, and we were in a position to bring them into line with the principles of the gastræa theory and trace them to the embryonic forms of the lower vertebrates. Although there is no independent food-yelk, apart from the formative yelk, in the mammal ovum, and although its segmentation is total on that account, nevertheless a large yelk-sac is formed in their embryos, and the “embryo proper” spreads leaf-wise over its surface, as in the reptiles and birds, which have a large food-yelk and partial segmentation. In the mammals, as well as in the latter, the flat, leaf-shaped germinal disk separates from the yelk-sac, and its edges join together and form tubes.
How can we explain this curious anomaly? Only as a result of very characteristic and peculiar cenogenetic modifications of the embryonic process, the real causes of which must be sought in the change in the rearing of the young on the part of the viviparous mammals. These are clearly connected with the fact that the ancestors of the viviparous mammals were oviparous amniotes like the present monotremes, and only gradually became viviparous. This can no longer be questioned now that it has been shown (1884) that the monotremes, the lowest and oldest of the mammals, still lay eggs, and that these develop like the ova of the reptiles and birds. Their nearest descendants, the marsupials, formed the habit of retaining the eggs, and developing them in the oviduct; the latter was thus converted into a womb (uterus). A nutritive fluid that was secreted from its wall, and passed through the wall of the blastula, now served to feed the embryo, and took the place of the food-yelk. In this way the original food-yelk of the monotremes gradually atrophied, and at last disappeared so completely that the partial ovum-segmentation of their descendants, the rest of the mammals, once more became total. From the discogastrula of the former was evolved the distinctive epigastrula of the latter.
It is only by this phylogenetic explanation that we can understand the formation and development of the peculiar, and hitherto totally misunderstood, blastula of the mammal. The vesicular condition of the mammal embryo was discovered 200 years ago (1677) by Regner de Graaf. He found in the uterus of a rabbit four days after impregnation small, round, loose, transparent vesicles, with a double envelope. However, Graaf’s discovery passed without recognition. It was not until 1827 that these vesicles were rediscovered by Baer, and then more closely studied in 1842 by Bischoff in the rabbit (Figs. 106, 107). They are found in the womb of the rabbit, the dog, and other small mammals, a few days after copulation. The mature ova of the mammal, when they have left the ovary, are fertilised either here or in the oviduct immediately afterwards by the invading sperm-cells.[25] (As to the womb and oviduct see Chapter XXIX) The cleavage and formation of the gastrula take place in the oviduct. Either here in the oviduct or after the mammal gastrula has passed into the uterus it is converted into the globular vesicle which is shown externally in Fig. 106, and in section in Fig. 107. The thick, outer, structureless envelope that encloses it is the original ovolemma or zona pellucida, modified, and clothed with a layer of albumin that has been deposited on the outside. From this stage the envelope is called the external membrane, the primary chorion or prochorion (a). The real wall of the vesicle enclosed by it consists of a simple layer of ectodermic cells (b), which are flattened by mutual pressure, and generally hexagonal; a light nucleus shines through their fine-grained protoplasm (Fig. 108). At one part (c) inside this hollow ball we find a circular disc, formed of darker, softer, and rounder cells, the dark-grained entodermic cells (Fig. 109).
[25] In man and the other mammals the fertilisation of the ova probably takes place, as a rule, in the oviduct; here the ova, which issue from the female ovary in the shape of the Graafian follicle, and enter the inner aperture of the oviduct, encounter the mobile sperm-cells of the male seed, which pass into the uterus at copulation, and from this into the external aperture of the oviduct. Impregnation rarely takes place in the ovary or in the womb.
Fig. 108—Four entodermic
cells from the embryonic vesicle of the rabbit.
Fig. 109—Two
entodermic cells from the embryonic vesicle of the rabbit.
The characteristic embryonic form that the developing mammal now exhibits has up to the present usually been called the “blastula” (Bischoff), “sac-shaped embryo” (Baer), “vesicular embryo” (vesicula blastodermica, or, briefly, blastosphæra). The wall of the hollow vesicle, which consists of a single layer of cells, was called the “blastoderm,” and was supposed to be equivalent to the cell-layer of the same name that forms the wall of the real blastula of the amphioxus and many of the invertebrates (such as Monoxenia, Fig. 29 F, G). Formerly this real blastula was generally believed to be equivalent to the embryonic vesicle of the mammal. However, this is by no means the case. What is called the “blastula” of the mammal and the real blastula of the amphioxus and many of the invertebrates are totally different embryonic structures. The latter (blastula) is palingenetic, and precedes the formation of the gastrula. The former (blastodermic vesicle) is cenogenetic, and follows gastrulation. The globular wall of the blastula is a real blastoderm, and consists of homogeneous (blastodermic) cells; it is not yet differentiated into the two primary germinal layers. But the globular wall of the mammal vesicle is the differentiated ectoderm, and at one point in it we find a circular disk of quite different cells—the entoderm. The round cavity, filled with fluid, inside the real blastula is the segmentation-cavity. But the similar cavity within the mammal vesicle is the yelk-sac cavity, which is connected with the incipient gut-cavity. This primitive gut-cavity passes directly into the segmentation-cavity in the mammals, in consequence of the peculiar cenogenetic changes in their gastrulation, which we have considered previously (Chapter IX). For these reasons it is very necessary to recognise the secondary embryonic vesicle in the mammal (gastrocystis or blastocystis) as a characteristic structure peculiar to this class, and distinguish it carefully from the primary blastula of the amphioxus and the invertebrates.
Fig. 110—Ovum of
a rabbit from the uterus, one sixth of an inch in diameter. The embryonic
vesicle (b) has withdrawn a little from the smooth ovolemma (a).
In the middle of the ovolemma we see the round germinal disk (blastodiscus,
c), at the edge of which (at d) the inner layer of the embryonic
vesicle is already beginning to expand. (Figs. 110–114 from
Bischoff.
Fig. 111—The same ovum, seen in profile.
Letters as in Fig. 110.
Fig. 112—Ovum of a rabbit from the
uterus, one-fourth of an inch in diameter. The blastoderm is already for
the most part two-layered (b). The ovolemma, or outer envelope, is
tufted (a).
Fig. 113—The same ovum, seen in profile.
Letters as in Fig. 112.
Fig. 114—Ovum of a rabbit from the
uterus, one-third of an inch in diameter. The embryonic vesicle is now
nearly everywhere two-layered (k) only remaining one-layered below (at
d).
The small, circular, whitish, and opaque spot which the gastric disk (Fig. 106) forms at a certain part of the surface of the clear and transparent embryonic vesicle has long been known to science, and compared to the germinal disk of the birds and reptiles. Sometimes it has been called the germinal disk, sometimes the germinal spot, and usually the germinative area. From the area the further development of the embryo proceeds. However, the larger part of the embryonic vesicle of the mammal is not directly used for building up the later body, but for the construction of the temporary umbilical vesicle. The embryo separates from this in proportion as it grows at its expense; the two are only connected by the yelk-duct (the stalk of the yelk-sac), and this maintains the direct communication between the cavity of the umbilical vesicle and the forming visceral cavity (Fig. 105).
Fig. 115—Round germinative area of the rabbit, divided
into the central light area (area pellucida) and the peripheral dark
area (area opaca). The light area seems darker on account of the dark
ground appearing through it.
Fig. 116—Oval area, with the
opaque whitish border of the dark area without.
The germinative area or gastric disk of the animal consists at first (like the germinal disk of birds and reptiles) merely of the two primary germinal layers, the ectoderm and entoderm. But soon there appears in the middle of the circular disk between the two a third stratum of cells, the rudiment of the middle layer or fibrous layer (mesoderm). This middle germinal layer consists from the first, as we have seen in Chapter X, of two separate epithelial plates, the two layers of the cœlom-pouches (parietal and visceral). However, in all the amniotes (on account of the large formation of yelk) these thin middle plates are so firmly pressed together that they seem to represent a single layer. It is thus peculiar to the amniotes that the middle of the germinative area is composed of four germinal layers, the two limiting (or primary) layers and the middle layers between them (Figs. 96, 97). These four secondary germinal layers can be clearly distinguished as soon as what is called the sickle-groove (or “embryonic sickle”) is seen at the hind border of the germinative area. At the borders, however, the germinative area of the mammal only consists of two layers. The rest of the wall of the embryonic vesicle consists at first (but only for a short time in most of the mammals) of a single layer, the outer germinal layer.
From this stage, however, the whole wall of the embryonic vesicle becomes two-layered. The middle of the germinative area is much thickened by the growth of the cells of the middle layers, and the inner layer expands at the same time, and increases at the border of the disk all round. Lying close on the outer layer throughout, it grows over its inner surface at all points, covers first the upper and then the lower hemisphere, and at last closes in the middle of the inner layer (Figs. 110–114). The wall of the embryonic vesicle now consists throughout of two layers of cells, the ectoderm without and the entoderm within. It is only in the centre of the circular area, which becomes thicker and thicker through the growth of the middle layers, that it is made up of all four layers. At the same time, small structureless tufts or warts are deposited on the surface of the outer ovolemma or prochorion, which has been raised above the embryonic vesicle (Figs. 112–114 a).
We may now disregard both the outer ovolemma and the greater part of the vesicle, and concentrate our attention on the germinative area and the four-layered embryonic disk. It is here alone that we find the important changes which lead to the differentiation of the first organs. It is immaterial whether we examine the germinative area of the mammal (the rabbit, for instance) or the germinal disk of a bird or a reptile (such as a lizard or tortoise). The embryonic processes we are now going to consider are essentially the same in all members of the three higher classes of vertebrates which we call the amniotes. Man is found to agree in this respect with the rabbit, dog, ox, etc.; and in all these animals the germinative area undergoes essentially the same changes as in the birds and reptiles. They are most frequently and accurately studied in the chick, because we can have incubated hens’ eggs in any quantity at any stage of development. Moreover, the round germinal disk of the chick passes immediately after the beginning of incubation (within a few hours) from the two-layered to the four-layered stage, the two-layered mesoderm developing from the median primitive groove between the ectoderm and entoderm (Figs. 82–95).
Fig. 117—Oval germinal disk of the rabbit,
magnified. As the delicate, half-transparent disk lies on a black ground, the
pellucid area looks like a dark ring, and the opaque area (lying outside it)
like a white ring. The oval shield in the centre also looks whitish, and in its
axis we see the dark medullary groove. (From Bischoff.)
Fig.
118—Pear-shaped germinal shield of the rabbit (eight days old),
magnified. rf medullary groove. pr primitive groove (primitive
mouth). (From Kölliker.
The first change in the round germinal disk of the chick is that the cells at its edges multiply more briskly, and form darker nuclei in their protoplasm. This gives rise to a dark ring, more or less sharply set off from the lighter centre of the germinal disk (Fig. 115). From this point the latter takes the name of the “light area” (area pellucida), and the darker ring is called the “dark area” (area opaca). (In a strong light, as in Figs. 115–117, the light area seems dark, because the dark ground is seen through it; and the dark area seems whiter). The circular shape of the area now changes into elliptic, and then immediately into oval (Figs. 116, 117). One end seems to be broader and blunter, the other narrower and more pointed; the former corresponds to the anterior and the latter to the posterior section of the subsequent body. At the same time, we can already trace the characteristic bilateral form of the body, the antithesis of right and left, before and behind. This will be made clearer by the “primitive streak,” which appears at the posterior end.
At an early stage an opaque spot is seen in the middle of the clear germinative area, and this also passes from a circular to an oval shape. At first this shield-shaped marking is very delicate and barely perceptible; but it soon becomes clearer, and now stands out as an oval shield, surrounded by two rings or areas (Fig. 117). The inner and brighter ring is the remainder of the pellucid area, and the dark outer ring the remainder of the opaque area; the opaque shield-like spot itself is the first rudiment of the dorsal part of the embryo. We give it briefly the name of embryonic shield or dorsal shield. In most works this embryonic shield is described as “the first rudiment or trace of the embryo,” or “primitive embryo.” But this is wrong, though it rests on the authority of Baer and Bischoff.
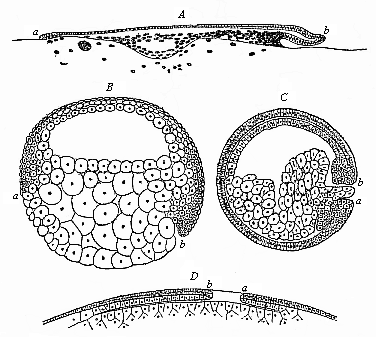
Fig. 119—Median longitudinal section of the gastrula of four vertebrates. (From Rabl.) A discogastrula of a shark (Pristiurus). B amphigastrula of a sturgeon (Accipenser). C amphigastrula of an amphibium (Triton). D epigastrula of an amniote (diagram). a ventral, b dorsal lip of the primitive mouth.
As a matter of fact, we already have the embryo in the stem-cell, the gastrula, and all the subsequent stages. The embryonic shield is simply the first rudiment of the dorsal part, which is the earliest to develop. As the older names of “embryonic rudiment” and “germinative area” are used in many different senses—and this has led to a fatal confusion in embryonic literature—we must explain very clearly the real significance of these important embryonic parts of the amniote. It will be useful to do so in a series of formal principles:—
1. The so-called ”first trace of the embryo” in the amniotes, or the embryonic shield, in the centre of the pellucid area, consists merely of an early differentiation and formation of the middle dorsal parts.
2. Hence the best name for it is ”the dorsal shield,” as I proposed long ago.
3. The germinative area, in which the first embryonic blood-vessels appear at an early stage, is not opposed as an external area to the ”embryo proper,” but is a part of it.
4. In the same way, the yelk-sac or the umbilical vesicle is not a foreign external appendage of the embryo, but an outlying part of its primitive gut.
5. The dorsal shield gradually separates from the germinative area and the yelk-sac, its edges growing downwards and folding together to form ventral plates.
6. The yelk-sac and vessels of the germinative area, which soon spread over its whole surface, are, therefore, real embryonic organs, or temporary parts of the embryo, and have a transitory importance in connection with the nutrition of the growing later body; the latter may be called the ”permanent body” in contrast to them.
The relation of these cenogenetic features of the amniotes to the palingenetic structures of the older non-amniotic vertebrates may be expressed in the following theses: The original gastrula, which completely passes into the embryonic body in the acrania, cyclostoma, and amphibia, is early divided into two parts in the amniotes—the embryonic shield, which represents the dorsal outline of the permanent body; and the temporary embryonic organs of the germinative area and its blood-vessels, which soon grow over the whole of the yelk-sac. The differences which we find in the various classes of the vertebrate stem in these important particulars can only be fully understood when we bear in mind their phylogenetic relations on the one hand, and, on the other, the cenogenetic modifications of structure that have been brought about by changes in the rearing of the young and the variation in the mass of the food-yelk.
We have already described in Chapter IX the changes which this increase and decrease of the nutritive yelk causes in the form of the gastrula, and especially in the situation and shape of the primitive mouth. The primitive mouth or prostoma is originally a simple round aperture at the lower pole of the long axis; its dorsal lip is above and ventral lip below. In the amphioxus this primitive mouth is a little eccentric, or shifted to the dorsal side (Fig. 39). The aperture increases with the growth of the food-yelk in the cyclostoma and ganoids; in the sturgeon it lies almost on the equator of the round ovum, the ventral lip (a) in front and the dorsal lip (b) behind (Fig. 119 b). In the wide-mouthed, circular discoid gastrula of the selachii or primitive fishes, which spreads quite flat on the large food-yelk, the anterior semi-circle of the border of the disk is the ventral, and the posterior semicircle the dorsal lip (Fig. 119 A). The amphiblastic amphibia are directly connected with their earlier fish-ancestors, the dipneusts and ganoids, and further the oldest selachii (Cestracion); they have retained their total unequal segmentation, and their small primitive mouth (Fig. 119 C, ab), blocked up by the yelk-stopper, lies at the limit of the dorsal and ventral surface of the embryo (at the lower pole of its equatorial axis), and there again has an upper dorsal and a lower ventral lip (a, b). The formation of a large food-yelk followed again in the stem-forms of the amniotes, the protamniotes or proreptilia, descended from the amphibia (Fig. 119 D). But here the accumulation of the food-yelk took place only in the ventral wall of the primitive-gut, so that the narrow primitive mouth lying behind was forced upwards, and came to lie on the back of the discoid ”epigastrula” in the shape of the ”primitive groove”; thus (in contrast to the case of the selachii, Fig. 119 A) the dorsal lip (b) had to be in front, and the ventral lip (a) behind (Fig. 119 D). This feature was transmitted to all the amniotes, whether they retained the large food-yelk (reptiles, birds, and monotremes), or lost it by atrophy (the viviparous mammals).
This phylogenetic explanation of gastrulation and cœlomation, and the comparative study of them in the various vertebrates, throw a clear and full light on many ontogenetic phenomena, as to which the most obscure and confused opinions were prevalent thirty years ago. In this we see especially the high scientific value of the biogenetic law and the careful separation of palingenetic from cenogenetic processes. To the opponents of this law the real explanation of these remarkable phenomena is impossible. Here, and in every other part of embryology, the true key to the solution lies in phylogeny.
The earliest stages of the human embryo are, for the reasons already given, either quite unknown or only imperfectly known to us. But as the subsequent embryonic forms in man behave and develop just as they do in all the other mammals, there cannot be the slightest doubt that the preceding stages also are similar. We have been able to see in the cœlomula of the human embryo (Fig. 97), by transverse sections through its primitive mouth, that its two cœlom-pouches are developed in just the same way as in the rabbit (Fig. 96); moreover, the peculiar course of the gastrulation is just the same.
Fig. 120—Embryonic vesicle of a seven-days-old rabbit with oval embryonic shield (ag). A seen from above, B from the side. (From Kölliker.) ag dorsal shield or embryonic spot. In B the upper half of the vesicle is made up of the two primary germinal layers, the lower (up to ge) only from the outer layer.
The germinative area forms in the human embryo in the same way as in the other mammals, and in the middle part of this we have the embryonic shield, the purport of which we considered in Chapter XII. The next changes in the embryonic disk, or the “embryonic spot,” take place in corresponding fashion. These are the changes we are now going to consider more closely.
The chief part of the oval embryonic shield is at first the narrow hinder end; it is in the middle line of this that the primitive streak appears (Fig. 121 ps).The narrow longitudinal groove in it—the so-called “primitive groove”—is, as we have seen, the primitive mouth of the gastrula. In the gastrula-embryos of the mammals, which are much modified cenogenetically, this cleft-shaped prostoma is lengthened so much that it soon traverses the whole of the hinder half of the dorsal shield; as we find in a rabbit embryo of six to eight days (Fig. 122 pr). The two swollen parallel borders that limit this median furrow are the side lips of the primitive mouth, right and left. In this way the bilateral-symmetrical type of the vertebrate becomes pronounced. The subsequent head of the amniote is developed from the broader and rounder fore-half of the dorsal shield.
In this fore-half of the dorsal shield a median furrow quickly makes its appearance (Fig. 123 rf). This is the broader dorsal furrow or medullary groove, the first beginning of the central nervous system. The two parallel dorsal or medullary swellings that enclose it grow together over it afterwards, and form the medullary tube. As is seen in transverse sections, it is formed only of the outer germinal layer (Figs. 95 and 136). The lips of the primitive mouth, however, lie, as we know, at the important point where the outer layer bends over the inner, and from which the two cœlom pouches grow between the primary germinal layers.
Fig. 121—Oval embryonic shield of the rabbit (A of six days eighteen hours, B of eight days). (From Kölliker.) ps primitive streak, pr primitive groove, arg area germinalis, sw sickle-shaped germinal growth.
Fig.
122—Dorsal shield (ag) and germinative area of a
rabbit-embryo of eight days. (From Kölliker.) pr primitive
groove, rf dorsal furrow.
Fig. 123.—Embryonic shield of a
rabbit of eight days. (From Van Beneden.) pr primitive
groove, cn canalis neurentericus, nk nodus neurentericus (or
“Hensen’s ganglion”), kf head-process
(chorda).
Thus the median primitive furrow (pr) in the hind-half and the median medullary furrow (Rf) in the fore-half of the oval shield are totally different structures, although the latter seems to a superficial observer to be merely the forward continuation of the former. Hence they were formerly always confused. This error was the more pardonable as immediately afterwards the two grooves do actually pass into each other in a very remarkable way. The point of transition is the remarkable neurenteric canal (Fig. 124 cn). But the direct connection which is thus established does not last long; the two are soon definitely separated by a partition.
Fig. 124—Longitudinal section of the cœlomula of amphioxus (from the left). i entoderm, d primitive gut, cn medullary duct, n nerve tube, m mesoderm, s first primitive segment, c cœlom-pouches. (From Hatschek.)
The enigmatic neurenteric canal is a very old embryonic organ, and of great phylogenetic interest, because it arises in the same way in all the chordonia (both tunicates and vertebrates). In every case it touches or embraces like an arch the posterior end of the chorda, which has been developed here in front out of the middle line of the primitive gut (between the two cœlom-folds of the sickle groove) (“head-process,” Fig. 123 kf). These very ancient and strictly hereditary structures, which have no physiological significance to-day, deserve (as “rudimentary organs”) our closest attention. The tenacity with which the useless neurenteric canal has been transmitted down to man through the whole series of vertebrates is of equal interest for the theory of descent in general, and the phylogeny of the chordonia in particular.
The connection which the neurenteric canal (Fig. 123 cn) establishes between the dorsal nerve-tube (n) and the ventral gut-tube (d) is seen very plainly in the amphioxus in a longitudinal section of the cœlomula, as soon as the primitive mouth is completely closed at its hinder end. The medullary tube has still at this stage an opening at the forward end, the neuroporus Fig. 83 np). This opening also is afterwards closed. There are then two completely closed canals over each other—the medullary tube above and the gastric tube below, the two being separated by the chorda. The same features as in the acrania are exhibited by the related tunicates, the ascidiæ.
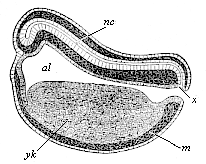
Fig. 125—Longitudinal section of the chordula of a frog. (From Balfour.) nc nerve-tube, x canalis neurentericus, al alimentary canal, yk yelk-cells, m mesoderm.
Again, we find the neurenteric canal in just the same form and situation in the amphibia. A longitudinal section of a young tadpole (Fig. 125) shows how we may penetrate from the still open primitive mouth (x) either into the wide primitive gut-cavity (al) or the narrow overlying nerve-tube. A little later, when the primitive mouth is closed, the narrow neurenteric canal (Fig. 126 ne) represents the arched connection between the dorsal medullary canal (mc) and the ventral gastric canal.
In the amniotes this original curved form of the neurenteric canal cannot be found at first, because here the primitive mouth travels completely over to the dorsal surface of the gastrula, and is converted into the longitudinal furrow we call the primitive groove. Hence the primitive groove (Fig. 128 pr), examined from above, appears to be the straight continuation of the fore-lying and younger medullary furrow (me). The divergent hind legs of the latter embrace the anterior end of the former. Afterwards we have the complete closing of the primitive mouth, the dorsal swellings joining to form the medullary tube and growing over it. The neurenteric canal then leads directly, in the shape of a narrow arch-shaped tube (Fig. 129 ne), from the medullary tube (sp) to the gastric tube (pag). Directly in front of it is the latter end of the chorda (cli).
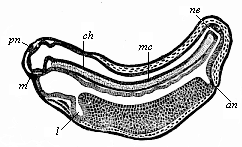
Fig. 126—Longitudinal section of a frog-embryo. (From Goette.) m mouth, l liver, an anus, ne canalis neurentericus, mc medullary-tube, pn pineal body (epiphysis), ch chorda.
While these important processes are taking place in the axial part of the dorsal shield, its external form also is changing. The oval form (Fig. 117) becomes like the sole of a shoe or sandal, lyre-shaped or finger-biscuit shaped (Fig. 130). The middle third does not grow in width as quickly as the posterior, and still less than the anterior third; thus the shape of the permanent body becomes somewhat narrow at the waist. At the same time, the oval form of the germinative area returns to a circular shape, and the inner pellucid area separates more clearly from the opaque outer area (Fig. 131 a). The completion of the circle in the area marks the limit of the formation of blood-vessels in the mesoderm.
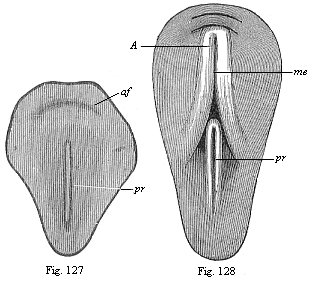
Figs. 127 and 128—Dorsal shield of the chick. (From Balfour.) The medullary furrow (me), which is not yet visible in Fig. 130, encloses with its hinder end the fore end of the primitive groove (pr) in Fig. 131.)
The characteristic sandal-shape of the dorsal shield, which is determined by the narrowness of the middle part, and which is compared to a violin, lyre, or shoe-sole, persists for a long time in all the amniotes. All mammals, birds, and reptiles have substantially the same construction at this stage, and even for a longer or shorter period after the division of the primitive segments into the cœlom-folds has begun (Fig. 132). The human embryonic shield assumes the sandal-form in the second week of development; towards the end of the week our sole-shaped embryo has a length of about one-twelfth of an inch (Fig. 133).
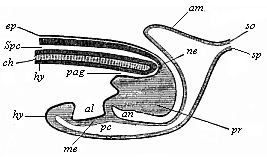
Fig. 129—Longitudinal section of the hinder end of a chick. (From Balfour.) sp medullary tube, connected with the terminal gut (pag) by the neurenteric canal (ne), ch chorda, pr neurenteric (or Hensen’s) ganglion, al allantois, ep ectoderm, hy entoderm, so parietal layer, sp visceral layer, an anus-pit, am amnion.
The complete bilateral symmetry of the vertebrate body is very early indicated in the oval form of the embryonic shield (Fig. 117) by the median primitive streak; in the sandal-form it is even more pronounced (Figs. 131–135). In the lateral parts of the embryonic shield a darker central and a lighter peripheral zone become more obvious; the former is called the stem-zone (Fig. 134 stz), and the latter the parietal zone (pz); from the first we get the dorsal and from the second the ventral half of the body-wall. The stem-zone of the amniote embryo would be called more appropriately the dorsal zone or dorsal shield; from it develops the whole of the dorsal half of the later body (or permanent body)—that is to say, the dorsal body (episoma). Again, it would be better to call the “parietal zone” the ventral zone or ventral shield; from it develop the ventral “lateral plates,” which afterwards separate from the embryonic vesicle and form the ventral body (hyposoma)—that is to say, the ventral half of the permanent body, together with the body-cavity and the gastric canal that it encloses.
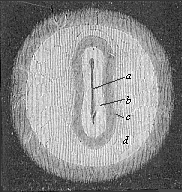
Fig. 130—Germinal area or germinal disk of the rabbit, with sole-shaped embryonic shield, magnified. The clear circular field (d) is the opaque area. The pellucid area (c) is lyre-shaped, like the embryonic shield itself (b). In its axis is seen the dorsal furrow or medullary furrow (a). (From Bischoff.
The sole-shaped germinal shields of all the amniotes are still, at the stage of construction which Fig. 134 illustrates in the rabbit and Fig. 135 in the opossum, so like each other that we can either not distinguish them at all or only by means of quite subordinate peculiarities in the size of the various parts. Moreover, the human sandal-shaped embryo cannot at this stage be distinguished from those of other mammals, and it particularly resembles that of the rabbit. On the other hand, the outer form of these flat sandal-shaped embryos is very different from the corresponding form of the lower animals, especially the acrania (amphioxus). Nevertheless, the body is just the same in the essential features of its structure as that we find in the chordula of the latter (Figs. 83–86), and in the embryonic forms which immediately develop from it. The striking external difference is here again due to the fact that in the palingenetic embryos of the amphioxus (Figs. 83, 84) and the amphibia (Figs. 85, 86) the gut-wall and body-wall form closed tubes from the first, whereas in the cenogenetic embryos of the amniotes they are forced to expand leaf-wise on the surface owing to the great extension of the food-yelk.
It is all the more notable that the early separation of dorsal and ventral halves takes place in the same rigidly hereditary fashion in all the vertebrates. In both the acrania and the craniota the dorsal body is about this period separated from the ventral body. In the middle part of the body this division has already taken place by the construction of the chorda between the dorsal nerve-tube and the ventral canal. But in the outer or lateral part of the body it is only brought about by the division of the coelom-pouches into two sections—a dorsal episomite (dorsal segment or provertebra) and a ventral hyposomite (or ventral segment) by a frontal constriction. In the amphioxus each of the former makes a muscular pouch, and each of the latter a sex-pouch or gonad.
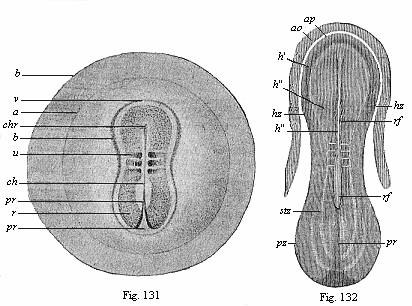
Fig. 131—Embryo of the opossum, sixty hours old,
one-sixth of an inch in diameter. (From Selenka) b the globular
embryonic vesicle, a the round germinative area, b limit of the
ventral plates, r dorsal shield, v its fore part, u the
first primitive segment, ch chorda, chr its fore-end, pr
primitive groove (or mouth).
Fig. 132—Sandal-shaped embryonic
shield of a rabbit of eight days, with the fore part of the germinative
area (ao opaque, ap pellucid area). (From Kölliker.)
rf dorsal furrow, in the middle of the medullary plate, h, pr
primitive groove (mouth), stz dorsal (stem) zone, pz ventral
(parietal) zone. In the narrow middle part the first three primitive segments
may be seen.
These important processes of differentiation in the mesoderm, which we will consider more closely in the next chapter, proceed step by step with interesting changes in the ectoderm, while the entoderm changes little at first. We can study these processes best in transverse sections, made vertically to the surface through the sole-shaped embryonic shield. Such a transverse section of a chick embryo, at the end of the first day of incubation, shows the gut-gland layer as a very simple epithelium, which is spread like a leaf over the outer surface of the food-yelk (Fig. 92). The chorda (ch) has separated from the dorsal middle line of the entoderm; to the right and left of it are the two halves of the mesoderm, or the two cœlom-folds. A narrow cleft in the latter indicates the body-cavity (uwh); this separates the two plates of the cœlom-pouches, the lower (visceral) and upper (parietal). The broad dorsal furrow (rf) formed by the medullary plate (m) is still wide open, but is divided from the lateral horn-plate (h) by the parallel medullary swellings, which eventually close.
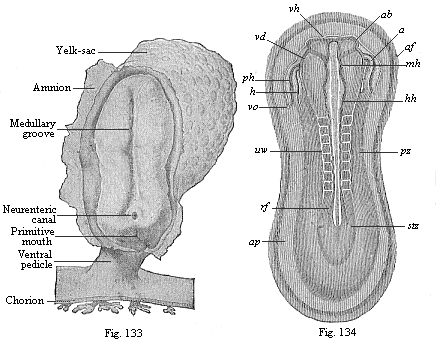
Fig. 133—Human embryo at the sandal-stage, one-twelfth of
an inch long, from the end of the second week, magnified. (From Count
Spee.)
Fig. 134—Sandal-shaped embryonic shield of a rabbit of
nine days. (From Kölliker.) (Back view from above.) stz
stem-zone or dorsal shield (with eight pairs of primitive segments), pz
parietal or ventral zone, ap pellucid area, af amnion-fold,
h heart, ph pericardial cavity, vo omphalo-mesenteric
vein, ab eye-vesicles, vh fore brain, mh middle brain,
hh hind brain, uw primitive segments (or vertebræ).
During these processes important changes are taking place in the outer germinal layer (the “skin-sense layer”). The continued rise and growth of the dorsal swellings causes their higher parts to bend together at their free borders, approach nearer and nearer (Fig. 136 w), and finally unite. Thus in the end we get from the open dorsal furrow, the upper cleft of which becomes narrower and narrower, a closed cylindrical tube (Fig. 137 mr). This tube is of the utmost importance; it is the beginning of the central nervous system, the brain and spinal marrow, the medullary tube. This embryonic fact was formerly looked upon as very mysterious. We shall see presently that in the light of the theory of descent it is a thoroughly natural process. The phylogenetic explanation of it is that the central nervous system is the organ by means of which all intercourse with the outer world, all psychic action and sense-perception, are accomplished; hence it was bound to develop originally from the outer and upper surface of the body, or from the outer skin. The medullary tube afterwards separates completely from the outer germinal layer, and is surrounded by the middle parts of the provertebræ and forced inwards (Fig. 146).The remaining portion of the skin-sense layer (Fig. 93 h) is now called the horn-plate or horn-layer, because from it is developed the whole of the outer skin or epidermis, with all its horny appendages (nails, hair, etc.).
A totally different organ, the prorenal (primitive kidney) duct (ung), is found to be developed at an early stage from the ectoderm. This is originally a quite simple, tube-shaped, lengthy duct, or straight canal, which runs from front to rear at each side of the provertebræ (on the outer side, Fig. 93 ung). It originates, it seems, out of the horn-plate at the side of the medullary tube, in the gap that we find between the provertebral and the lateral plates. The prorenal duct is visible in this gap even at the time of the severance of the medullary tube from the horn-plate. Other observers think that the first trace of it does not come from the skin-sense layer, but the skin-fibre layer.
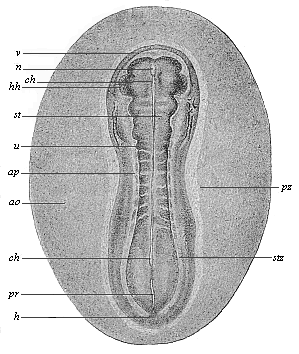
Fig. 135—Sandal-shaped embryonic shield of an opossum (Didelphys), three days old. (From Selenka.) (Back view from above.) stz stem-zone or dorsal shield (with eight pairs of primitive segments), pz parietal or ventral zone, ap pellucid area, ao opaque area, hh halves of the heart, v fore-end, h hind-end. In the median line we see the chorda (ch) through the transparent medullary tube (m). u primitive segment, pr primitive streak (or primitive mouth).
The inner germinal layer, or the gut-fibre layer (Fig. 93 dd), remains unchanged during these processes. A little later, however, it shows a quite flat, groove-like depression in the middle line of the embryonic shield, directly under the chorda. This depression is called the gastric groove or furrow. This at once indicates the future lot of this germinal layer. As this ventral groove gradually deepens, and its lower edges bend towards each other, it is formed into a closed tube, the alimentary canal, in the same way as the medullary groove grows into the medullary tube. The gut-fibre layer (Fig. 137 f), which lies on the gut-gland layer (d), naturally follows it in its folding. Moreover, the incipient gut-wall consists from the first of two layers, internally the gut-gland layer and externally the gut-fibre layer.
The formation of the alimentary canal resembles that of the medullary tube to this extent—in both cases a straight groove or furrow arises first of all in the middle line of a flat layer. The edges of this furrow then bend towards each other, and join to form a tube (Fig. 137). But the two processes are really very different. The medullary tube closes in its whole length, and forms a cylindrical tube, whereas the alimentary canal remains open in the middle, and its cavity continues for a long time in connection with the cavity of the embryonic vesicle. The open connection between the two cavities is only closed at a very late stage, by the construction of the navel. The closing of the medullary tube is effected from both sides, the edges of the groove joining together from right and left. But the closing of the alimentary canal is not only effected from right and left, but also from front and rear, the edges of the ventral groove growing together from every side towards the navel. Throughout the three higher classes of vertebrates the whole of this process of the construction of the gut is closely connected with the formation of the navel, or with the separation of the embryo from the yelk-sac or umbilical vesicle.
In order to get a clear idea of this, we must understand carefully the relation of the embryonic shield to the germinative area and the embryonic vesicle. This is done best by a comparison of the five stages which are shown in longitudinal section in Figs. 138–142. The embryonic shield (c), which at first projects very slightly over the surface of the germinative area, soon begins to rise higher above it, and to separate from the embryonic vesicle. At this point the embryonic shield, looked at from the dorsal surface, shows still the original simple sandal-shape (Figs. 133–135). We do not yet see any trace of articulation into head, neck, trunk, etc., or limbs. But the embryonic shield has increased greatly in thickness, especially in the anterior part. It now has the appearance of a thick, oval swelling, strongly curved over the surface of the germinative area. It begins to sever completely from the embryonic vesicle, with which it is connected at the ventral surface. As this severance proceeds, the back bends more and more; in proportion as the embryo grows the embryonic vesicle decreases, and at last it merely hangs as a small vesicle from the belly of the embryo (Fig. 142 ds). In consequence of the growth-movements which cause this severance, a groove-shaped depression is formed at the surface of the vesicle, the limiting furrow, which surrounds the vesicle in the shape of a pit, and a circular mound or dam (Fig. 139 ks) is formed at the outside of this pit by the elevation of the contiguous parts of the germinal vesicle.
Fig. 136—Transverse section of the embryonic disk of a chick at the end of the first day of incubation, magnified. The edges of the medullary plate (m), the medullary swellings (w), which separate the medullary from the horn-plate (h), are bending towards each other. At each side of the chorda (ch) the primitive segment plates (u) have separated from the lateral plates (sp). A gut-gland layer. (From Remak.)
In order to understand clearly this important process, we may compare the embryo to a fortress with its surrounding rampart and trench. The ditch consists of the outer part of the germinative area, and comes to an end at the point where the area passes into the vesicle. The important fold of the middle germinal layer that brings about the formation of the body-cavity spreads beyond the borders of the embryo over the whole germinative area. At first this middle layer reaches as far as the germinative area; the whole of the rest of the embryonic vesicle consists in the beginning only of the two original limiting layers, the outer and inner germinal layers. Hence, as far as the germinative area extends the germinal layer splits into the two plates we have already recognised in it, the outer skin-fibre layer and the inner gut-fibre layer. These two plates diverge considerably, a clear fluid gathering between them (Fig. 140 am). The inner plate, the gut-fibre layer, remains on the inner layer of the embryonic vesicle (on the gut-gland layer). The outer plate, the skin-fibre layer, lies close on the outer layer of the germinative area, or the skin-sense layer, and separates together with this from the embryonic vesicle. From these two united outer plates is formed a continuous membrane. This is the circular mound that rises higher and higher round the whole embryo, and at last joins above it (Figs. 139–142 am). To return to our illustration of the fortress, we must imagine the circular rampart to be extraordinarily high and towering far above the fortress. Its edges bend over like the combs of an overhanging wall of rock that would enclose the fortress; they form a deep hollow, and at last join together above. In the end the fortress lies entirely within the hollow that has been formed by the growth of the edges of this large rampart.
Fig. 137—Three diagrammatic transverse sections of the embryonic disk of the higher vertebrate, to show the origin of the tubular organs from the bending germinal layers. In Fig. A the medullary tube (n) and the alimentary canal (a) are still open grooves. In Fig. B the medullary tube (n) and the dorsal wall are closed, but the alimentary canal (a) and the ventral wall are open; the prorenal ducts (u) are cut off from the horn-plate (h) and internally connected with segmental prorenal canals. In Fig. C both the medullary tube and the dorsal wall above and the alimentary canal and ventral wall below are closed. All the open grooves have become closed tubes; the primitive kidneys are directed inwards. The letters have the same meaning in all three figures: h skin-sense layer, n medullary tube, u prorenal ducts, x axial rod, s primitive-vertebra, r dorsal wall, b ventral wall, c body-cavity or cœloma, f gut-fibre layer, t primitive artery (aorta), v primitive vein (subintestinal vein), d gut-fibre layer, a alimentary canal.
As the two outer layers of the germinative area thus rise in a fold about the embryo, and join above it, they come at last to form a spacious sac-like membrane about it. This envelope takes the name of the germinative membrane, or water-membrane, or amnion (Fig. 142 am). The embryo floats in a watery fluid, which fills the space between the embryo and the amnion, and is called the amniotic fluid (Figs. 141, 142 ah). We will deal with this remarkable formation and with the allantois later on (Chapter XV). In front of the allantois the yelk-sac or umbilical vesicle (ds), the remainder of the original embryonic vesicle, starts from the open belly of the embryo (Fig. 138 kh). In more advanced embryos, in which the gastric wall and the ventral wall are nearly closed, it hangs out of the navel-opening in the shape of a small vesicle with a stalk (Figs. 141, 142 ds). The more the embryo grows, the smaller becomes the vitelline (yelk) sac. At first the embryo looks like a small appendage of the large embryonic vesicle. Afterwards it is the yelk-sac, or the remainder of the embryonic vesicle, that seems a small pouch-like appendage of the embryo (Fig. 142 ds). It ceases to have any significance in the end. The very wide opening, through which the gastric cavity at first communicates with the umbilical vesicle, becomes narrower and narrower, and at last disappears altogether. The navel, the small pit-like depression that we find in the developed man in the middle of the abdominal wall, is the spot at which the remainder of the embryonic vesicle (the umbilical vesicle) originally entered into the ventral cavity, and joined on to the growing gut.
The origin of the navel coincides with the complete closing of the external ventral wall. In the amniotes the ventral wall originates in the same way as the dorsal wall. Both are formed substantially from the skin-fibre layer, and externally covered with the horn-plate, the border section of the skin-sense layer. Both come into existence by the conversion of the four flat germinal layers of the embryonic shield into a double tube by folding from opposite directions; above, at the back, we have the vertebral canal which encloses the medullary tube, and below, at the belly, the wall of the body-cavity which contains the alimentary canal (Fig. 137).
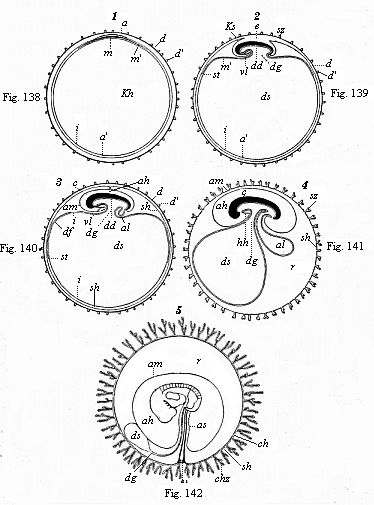
Figs. 138–142—Five diagrammatic longitudinal sections of the maturing mammal embryo and its envelopes. In Figs. 138–141 the longitudinal section passes through the sagittal or middle plane of the body, dividing the right and left halves; in Fig. 142 the embryo is seen from the left side. In Fig. 138 the tufted it prochorion (dd′) encloses the germinal vesicle, the wall of which consists of the two primary layers. Between the outer (a) and inner (i) layer the middle layer (m) has been developed in the region of the germinative area. In Fig. 139 the embryo (e) begins to separate from the embryonic vesicle (ds), while the wall of the amnion-fold rises about it (in front as head-sheath, ks, behind as tail-sheath, ss). In Fig. 140 the edges of the amniotic fold (am) rise together over the back of the embryo, and form the amniotic cavity (ah); as the embryo separates more completely from the embryonic vesicle (ds) the alimentary canal (dd) is formed, from the hinder end of which the allantois grows (al). In Fig. 141 the allantois is larger; the yelk-sac (ds) smaller. In Fig. 142 the embryo shows the gill-clefts and the outline of the two legs; the chorion has formed branching villi (tufts.) In all four figures e=embryo, a outer germinal layer, m middle germinal layer, i inner germinal layer, am amnion (ks head-sheath, ss tail-sheath), ah amniotic cavity, as amniotic sheath of the umbilical cord, kh embryonic vesicle, ds yelk-sac (umbilical vesicle), dg vitelline duct, df gut-fibre layer, dd gut-gland layer, al allantois, vl=hh place of heart, d vitelline membrane (ovolemma or prochorion), d′ tufts or villi of same, sh serous membrane (serolemma), sz tufts of same, ch chorion, chz tufts or villi, st terminal vein, r pericœlom or serocœlom (the space, filled with fluid, between the amnion and chorion). (From Kölliker.)
Figs. 143–144—Transverse sections of embryos (of chicks). Fig. 143 of the second, Fig. 144 of the third, Fig. 145 of the fourth, and Fig. 146 of the fifth day of incubation. Fig. 143–145 from Kölliker, magnified; Fig. 146 from Remak, magnified. h horn-plate, mr medullary tube, ung prorenal duct, un prorenal vesicles, hp skin-fibre layer, m=mu=mp muscle-plate, uw provertebral plate (wh cutaneous rudiment of the body of the vertebra, wb of the arch of the vertebra, wq the rib or transverse continuation), uwh provertebral cavity, ch axial rod or chorda, sh chorda-sheath, bh ventral wall, g hind and v fore root of the spinal nerves, a=af=am amniotic fold, p body-cavity or cœloma, df gut-fibre layer, ao primitive aortas, sa secondary aorta, vc cardinal veins, d=dd gut-gland layer, dr gastric groove. In Fig. 143 the larger part of the right half, in Fig. 144 the larger part of the left half, of the section is omitted. Of the yelk-sac or remainder of the embryonic vesicle only a small piece of the wall is indicated below.
We will consider the formation of the dorsal wall first, and that of the ventral wall afterwards (Figs. 143–147). In the middle of the dorsal surface of the embryo there is originally, as we already know, the medullary (mr) tube directly underneath the horn-plate (h), from the middle part of which it has been developed. Later, however, the provertebral plates (uw) grow over from the right and left between these originally connected parts (Figs. 145, 146). The upper and inner edges of the two provertebral plates push between the horn-plate and medullary tube, force them away from each other, and finally join between them in a seam that corresponds to the middle line of the back. The coalescence of these two dorsal plates and the closing in the middle of the dorsal wall take place in the same way as the medullary tube, which is henceforth enclosed by the vertebral tube. Thus is formed the dorsal wall, and the medullary tube takes up a position inside the body. In the same way the provertebral mass grows afterwards round the chorda, and forms the vertebral column. Below this the inner and outer edge of the provertebral plate splits on each side into two horizontal plates, of which the upper pushes between the chorda and medullary tube, and the lower between the chorda and gastric tube. As the plates meet from both sides above and below the chorda, they completely enclose it, and so form the tubular, outer chord-sheath, the sheath from which the vertebral column is formed (perichorda, Fig. 137 C, s; Figs. 145 uwh, 146).
We find in the construction of the ventral wall precisely the same processes as in the formation of the dorsal wall (Fig. 137 B, Fig. 144 hp, Fig. 146 bh). It is formed on the flat embryonic shield of the amniotes from the upper plates of the parietal zone. The right and left parietal plates bend downwards towards each other, and grow round the gut in the same way as the gut itself closes. The outer part of the lateral plates forms the ventral wall or the lower wall of the body, the two lateral plates bending considerably on the inner side of the amniotic fold, and growing towards each other from right and left. While the alimentary canal is closing, the body-wall also closes on all sides. Hence the ventral wall, which encloses the whole ventral cavity below, consists of two parts, two lateral plates that bend towards each other. These approach each other all along, and at last meet at the navel. We ought, therefore, really to distinguish two navels, an inner and an outer one. The internal or intestinal navel is the definitive point of the closing of the gut wall, which puts an end to the open communication between the ventral cavity and the cavity of the yelk-sac (Fig. 105). The external navel in the skin is the definitive point of the closing of the ventral wall; this is visible in the developed body as a small depression.
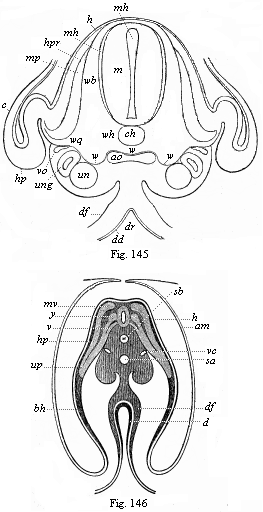
Figs. 145–146—Transverse sections of embryos (of chicks). Fig. 143 of the second, Fig. 144 of the third, Fig. 145 of the fourth, and Fig. 146 of the fifth day of incubation. Fig. 143–145 from Kölliker, magnified; Fig. 146 from Remak, magnified. h horn-plate, mr medullary tube, ung prorenal duct, un prorenal vesicles, hp skin-fibre layer, m=mu=mp muscle-plate, uw provertebral plate (wh cutaneous rudiment of the body of the vertebra, wb of the arch of the vertebra, wq the rib or transverse continuation), uwh provertebral cavity, ch axial rod or chorda, sh chorda-sheath, bh ventral wall, g hind and v fore root of the spinal nerves, a=af=am amniotic fold, p body-cavity or cœloma, df gut-fibre layer, ao primitive aortas, sa secondary aorta, vc cardinal veins, d=dd gut-gland layer, dr gastric groove. In Fig. 143 the larger part of the right half, in Fig. 144 the larger part of the left half, of the section is omitted. Of the yelk-sac or remainder of the embryonic vesicle only a small piece of the wall is indicated below.
With the formation of the internal navel and the closing of the alimentary canal is connected the formation of two cavities, which we call the capital and the pelvic sections of the visceral cavity. As the embryonic shield lies flat on the wall of the embryonic vesicle at first, and only gradually separates from it, its fore and hind ends are independent in the beginning; on the other hand, the middle part of the ventral surface is connected with the yelk-sac by means of the vitelline or umbilical duct (Fig. 147 m). This leads to a notable curving of the dorsal surface; the head-end bends downwards towards the breast and the tail-end towards the belly. We see this very clearly in the excellent old diagrammatic illustration given by Baer (Fig. 147), a median longitudinal section of the embryo of the chick, in which the dorsal body or episoma is deeply shaded. The embryo seems to be trying to roll up, like a hedgehog protecting itself from its pursuers. This pronounced curve of the back is due to the more rapid growth of the convex dorsal surface, and is directly connected with the severance of the embryo from the yelk-sac. The further bending of the embryo leads to the formation of the “head-cavity” of the gut (Fig. 148 above D) and a similar one at the tail, known as its “pelvic cavity.”
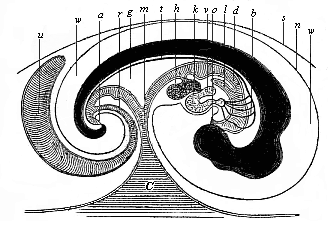
Fig. 147—Median longitudinal section of the embryo of a chick (fifth day of incubation), seen from the right side (head to the right, tail to the left). Dorsal body dark, with convex outline. d gut, o mouth, a anus, l lungs, h liver, g mesentery, v auricle of the heart, k ventricle of the heart, b arch of the arteries, t aorta, c yelk-sac, m vitelline (yelk) duct, u allantois, r pedicle (stalk) of the allantois, n amnion, w amniotic cavity, s serous membrane. (From Baer.)
As a result of these processes the embryo attains a shape that may be compared to a wooden shoe, or, better still, to an overturned canoe. Imagine a canoe or boat with both ends rounded and a small covering before and behind; if this canoe is turned upside down, so that the curved keel is uppermost, we have a fair picture of the canoe-shaped embryo (Fig. 147). The upturned convex keel corresponds to the middle line of the back; the small chamber underneath the fore-deck represents the capital cavity, and the small chamber under the rear-deck the pelvic chamber of the gut (cf. Fig. 140).
The embryo now, as it were, presses into the outer surface of the embryonic vesicle with its free ends, while it moves away from it with its middle part. As a result of this change the yelk-sac becomes henceforth only a pouch-like outer appendage at the middle of the ventral wall. The ventral appendage, growing smaller and smaller, is afterwards called the umbilical (navel) vesicle. The cavity of the yelk-sac or umbilical vesicle communicates with the corresponding visceral cavity by a wide opening, which gradually contracts into a narrow and long canal, the vitelline (yelk) duct (ductus vitellinus, Fig. 147 m). Hence, if we were to imagine ourselves in the cavity of the yelk-sac, we could get from it through the yelk-duct into the middle and still wide open part of the alimentary canal. If we were to go forward from there into the head-part of the embryo, we should reach the capital cavity of the gut, the fore-end of which is closed up.
The reader will ask: “Where are the mouth and the anus?” These are not at first present in the embryo. The whole of the primitive gut-cavity is completely closed, and is merely connected in the middle by the vitelline duct with the equally closed cavity of the embryonic vesicle (Fig. 140). The two later apertures of the alimentary canal—the anus and the mouth—are secondary constructions, formed from the outer skin. In the horn-plate, at the spot where the mouth is found subsequently, a pit-like depression is formed, and this grows deeper and deeper, pushing towards the blind fore-end of the capital cavity; this is the mouth-pit. In the same way, at the spot in the outer skin where the anus is afterwards situated a pit-shaped depression appears, grows deeper and deeper, and approaches the blind hind-end of the pelvic cavity; this is the anus-pit. In the end these pits touch with their deepest and innermost points the two blind ends of the primitive alimentary canal, so that they are now only separated from them by thin membranous partitions. This membrane finally disappears, and henceforth the alimentary canal opens in front at the mouth and in the rear by the anus (Figs. 141, 147). Hence at first, if we penetrate into these pits from without, we find a partition cutting them off from the cavity of the alimentary canal, which gradually disappears. The formation of mouth and anus is secondary in all the vertebrates.
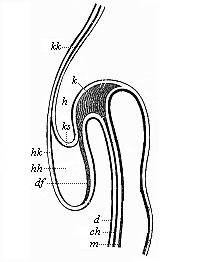
Fig. 148—Longitudinal section of the fore half of a chick-embryo at the end of the first day of incubation (seen from the left side). k head-plates, ch chorda. Above it is the blind fore-end of the ventral tube (m); below it the capital cavity of the gut. d gut-gland layer, df gut-fibre layer, h horn plate, hh cavity of the heart, hk heart-capsule, ks head-sheath, kk head-capsule. (From Remak.)
During the important processes which lead to the formation of the navel, and of the intestinal wall and ventral wall, we find a number of other interesting changes taking place in the embryonic shield of the amniotes. These relate chiefly to the prorenal ducts and the first blood-vessels. The prorenal (primitive kidney) ducts, which at first lie quite flat under the horn-plate or epiderm (Fig. 93 ung), soon back towards each other in consequence of special growth movements (Figs. 143–145 ung). They depart more and more from their point of origin, and approach the gut-gland layer. In the end they lie deep in the interior, on either side of the mesentery, underneath the chorda, (Fig. 145 ung). At the same time, the two primitive aortas change their position (cf. Figs. 138–145 ao); they travel inwards underneath the chorda, and there coalesce at last to form a single secondary aorta, which is found under the rudimentary vertebral column (Fig. 145 ao). The cardinal veins, the first venous blood-vessels, also back towards each other, and eventually unite immediately above the rudimentary kidneys (Figs. 145 vc, 152 cav). In the same spot, at the inner side of the fore-kidneys, we soon see the first trace of the sexual organs. The most important part of this apparatus (apart from all its appendages) is the ovary in the female and the testicle in the male. Both develop from a small part of the cell-lining of the body-cavity, at the spot where the skin-fibre layer and gut-fibre layer touch. The connection of this embryonic gland with the prorenal ducts, which lie close to it and assume most important relations to it, is only secondary.
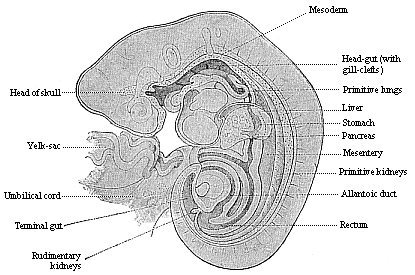
Fig. 149—Longitudinal section of a human embryo of the fourth week, one-fifth of an inch long, magnified. (From Kollmann.)
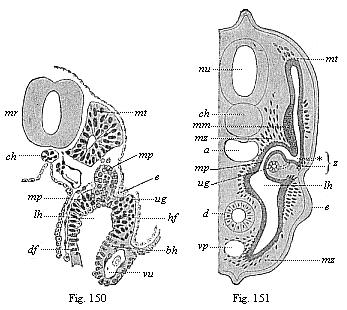
Fig.
150—Transverse section of a human embryo of fourteen days.
mr medullary tube, ch chorda. vu umbilical vein, mt
myotome, mp middle plate, ug prorenal duct, lh
body-cavity, e ectoderm, bh ventral skin, hf skin-fibre
layer, df gut-fibre layer. (From Kollmann.)
Fig. 151—Transverse section of a
shark-embryo (or young selachius). mr medullary tube, ch
chorda, a aorta, d gut, vp principal (or subintestinal)
vein, mt myotome, mm muscular mass of the provertebra, mp
middle plate, ug prorenal duct, lh body-cavity, e ectoderm
of the rudimentary extremities, mz mesenchymic cells, z point
where the myotome and nephrotome separate. (From H. E.
Ziegler.)
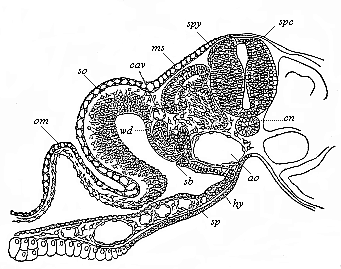
Fig. 152—Transverse section of a duck-embryo with twenty-four primitive segments. (From Balfour.) From a dorsal lateral joint of the medullary tube (spc) the spinal ganglia (spg) grow out between it and the horn-plate. ch chorda, ao double aorta, hy gut-gland layer, sp gut-fibre layer, with blood-vessels in section, ms muscle plate, in the dorsal wall of the myocœl (episomite). Below the cardinal vein (cav) is the prorenal duct (wd) and a segmental prorenal canal (st). The skin-fibre layer of the body-wall (so) is continued in the amniotic fold (am). Between the four secondary germinal layers and the structures formed from them there is formed embryonic connective matter with stellate cells and vascular structures (Hertwig’s “mesenchym”).
[26] The term articulation is used in this chapter to denote both “segmentation” and “articulation” in the ordinary sense.—Translator.
The vertebrate stem, to which our race belongs as one of the latest and most advanced outcomes of the natural development of life, is rightly placed at the head of the animal kingdom. This privilege must be accorded to it, not only because man does in point of fact soar far above all other animals, and has been lifted to the position of “lord of creation”; but also because the vertebrate organism far surpasses all the other animal-stems in size, in complexity of structure, and in the advanced character of its functions. From the point of view of both anatomy and physiology, the vertebrate stem outstrips all the other, or invertebrate, animals.
There is only one among the twelve stems of the animal kingdom that can in many respects be compared with the vertebrates, and reaches an equal, if not a greater, importance in many points. This is the stem of the articulates, composed of three classes: 1, the annelids (earth-worms, leeches, and cognate forms); 2, the crustacea (crabs, etc.); 3, the tracheata (spiders, insects, etc.). The stem of the articulates is superior not only to the vertebrates, but to all other animal-stems, in variety of forms, number of species, elaborateness of individuals, and general importance in the economy of nature.
When we have thus declared the vertebrates and the articulates to be the most important and most advanced of the twelve stems of the animal kingdom, the question arises whether this special position is accorded to them on the ground of a peculiarity of organisation that is common to the two. The answer is that this is really the case; it is their segmental or transverse articulation, which we may briefly call metamerism. In all the vertebrates and articulates the developed individual consists of a series of successive members (segments or metamera = “parts”); in the embryo these are called primitive segments or somites. In each of these segments we have a certain group of organs reproduced in the same arrangement, so that we may regard each segment as an individual unity, or a special “individual” subordinated to the entire personality.
The similarity of their segmentation, and the consequent physiological advance in the two stems of the vertebrates and articulates, has led to the assumption of a direct affinity between them, and an attempt to derive the former directly from the latter. The annelids were supposed to be the direct ancestors, not only of the crustacea and tracheata, but also of the vertebrates. We shall see later (Chapter XX) that this annelid theory of the vertebrates is entirely wrong, and ignores the most important differences in the organisation of the two stems. The internal articulation of the vertebrates is just as profoundly different from the external metamerism of the articulates as are their skeletal structure, nervous system, vascular system, and so on. The articulation has been developed in a totally different way in the two stems. The unarticulated chordula (Figs. 83–86), which we have recognised as one of the chief palingenetic embryonic forms of the vertebrate group, and from which we have inferred the existence of a corresponding ancestral form for all the vertebrates and tunicates, is quite unthinkable as the stem-form of the articulates.
All articulated animals came originally from unarticulated ones. This phylogenetic principle is as firmly established as the ontogenetic fact that every articulated animal-form develops from an unarticulated embryo. But the organisation of the embryo is totally different in the two stems. The chordula-embryo of all the vertebrates is characterised by the dorsal medullary tube, the neurenteric canal, which passes at the primitive mouth into the alimentary canal, and the axial chorda between the two. None of the articulates, either annelids or arthropods (crustacea and tracheata), show any trace of this type of organisation. Moreover, the development of the chief systems of organs proceeds in the opposite way in the two stems. Hence the segmentation must have arisen independently in each. This is not at all surprising; we find analogous cases in the stalk-articulation of the higher plants and in several groups of other animal stems.
The characteristic internal articulation of the vertebrates and its importance in the organisation of the stem are best seen in the study of the skeleton. Its chief and central part, the cartilaginous or bony vertebral column, affords an obvious instance of vertebrate metamerism; it consists of a series of cartilaginous or bony pieces, which have long been known as vertebræ (or spondyli). Each vertebra is directly connected with a special section of the muscular system, the nervous system, the vascular system, etc. Thus most of the “animal organs” take part in this vertebration. But we saw, when we were considering our own vertebrate character (in Chapter XI), that the same internal articulation is also found in the lowest primitive vertebrates, the acrania, although here the whole skeleton consists merely of the simple chorda, and is not at all articulated. Hence the articulation does not proceed primarily from the skeleton, but from the muscular system, and is clearly determined by the more advanced swimming-movements of the primitive chordonia-ancestors.
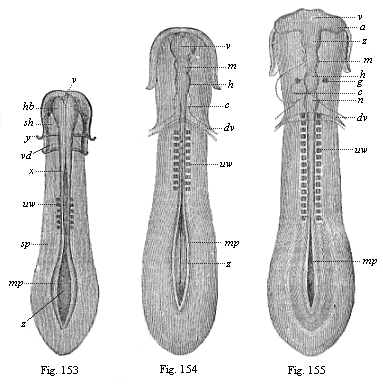
Figs. 153–155—Sole-shaped embryonic disk of the chick, in three successive stages of development, looked at from the dorsal surface, magnified, somewhat diagrammatic. Fig. 153 with six pairs of somites. Brain a simple vesicle (hb). Medullary furrow still wide open from x; greatly widened at z. mp medullary plates, sp lateral plates, y limit of gullet-cavity (sh) and fore-gut (vd). Fig. 154 with ten pairs of somites. Brain divided into three vesicles: v fore-brain, m middle-brain, h hind-brain, c heart, dv vitelline-veins. Medullary furrow still wide open behind (z). mp medullary plates. Fig. 155 with sixteen pairs of somites. Brain divided into five vesicles: v fore-brain, z intermediate-brain, m middle-brain, h hind-brain, n after-brain, a optic vesicles, g auditory vesicles, c heart, dv vitelline veins, mp medullary plate, uw primitive vertebra.
It is, therefore, wrong to describe the first rudimentary segments in the vertebrate embryo as primitive vertebræ or provertebræ; the fact that they have been so called for some time has led to much error and misunderstanding. Hence we shall give the name of “somites” or primitive segments to these so-called “primitive vertebræ.” If the latter name is retained at all, it should only be used of the sclerotom—i.e., the small part of the somites from which the later vertebra does actually develop.
Articulation begins in all vertebrates at a very early embryonic stage, and this indicates the considerable phylogenetic age of the process. When the chordula (Figs. 83–86) has completed its characteristic composition, often even a little earlier, we find in the amniotes, in the middle of the sole-shaped embryonic shield, several pairs of dark square spots, symmetrically distributed on both sides of the chorda (Figs. 131–135).Transverse sections (Fig. 93 uw) show that they belong to the stem-zone (episoma) of the mesoderm, and are separated from the parietal zone (hyposoma) by the lateral folds; in section they are still quadrangular, almost square, so that they look something like dice. These pairs of “cubes” of the mesoderm are the first traces of the primitive segments or somites, the so-called “protovertebræ.” (Figs. 153–155 uw).
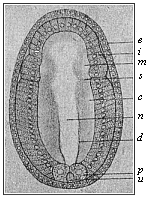
Fig. 156—Embryo of the amphioxus, sixteen hours old, seen from the back. (From Hatschek.) d primitive gut, u primitive mouth, p polar cells of the mesoderm, c cœlom-pouches, m their first segment, n medullary tube, i entoderm, e ectoderm, s first segment-fold.
Among the mammals the embryos of the marsupials have three pairs of somites (Fig. 131) after sixty hours, and eight pairs after seventy-two hours (Fig. 135). They develop more slowly in the embryo of the rabbit; this has three somites on the eighth day (Fig. 132), and eight somites a day later (Fig. 134). In the incubated hen’s egg the first somites make their appearance thirty hours after incubation begins (Fig. 153). At the end of the second day the number has risen to sixteen or eighteen (Fig. 155). The articulation of the stem-zone, to which the somites owe their origin, thus proceeds briskly from front to rear, new transverse constrictions of the “protovertebral plates” forming continuously and successively. The first segment, which is almost half-way down in the embryonic shield of the amniote, is the foremost of all; from this first somite is formed the first cervical vertebra with its muscles and skeletal parts. It follows from this, firstly, that the multiplication of the primitive segments proceeds backwards from the front, with a constant lengthening of the hinder end of the body; and, secondly, that at the beginning of segmentation nearly the whole of the anterior half of the sole-shaped embryonic shield of the amniote belongs to the later head, while the whole of the rest of the body is formed from its hinder half. We are reminded that in the amphioxus (and in our hypothetic primitive vertebrate, Figs. 98–102) nearly the whole of the fore half corresponds to the head, and the hind half to the trunk.
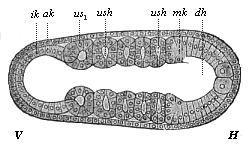
Fig. 157—Embryo of the amphioxus, twenty hours old, with five somites. (Right view; for left view see Fig. 124.) (From Hatschek.) V fore end, H hind end. ak, mk, ik outer, middle, and inner germinal layers; dh alimentary canal, n neural tube, cn canalis neurentericus, ush cœlom-pouches (or primitive-segment cavities), us1 first (and foremost) primitive segment.
The number of the metamera, and of the embryonic somites or primitive segments from which they develop, varies considerably in the vertebrates, according as the hind part of the body is short or is lengthened by a tail. In the developed man the trunk (including the rudimentary tail) consists of thirty-three metamera, the solid centre of which is formed by that number of vertebræ in the vertebral column (seven cervical, twelve dorsal, five lumbar, five sacral, and four caudal). To these we must add at least nine head-vertebræ, which originally (in all the craniota) constitute the skull. Thus the total number of the primitive segments of the human body is raised to at least forty-two; it would reach forty-five to forty-eight if (according to recent investigations) the number of the original segments of the skull is put at twelve to fifteen. In the tailless or anthropoid apes the number of metamera is much the same as in man, only differing by one or two; but it is much larger in the long-tailed apes and most of the other mammals. In long serpents and fishes it reaches several hundred (sometimes 400).
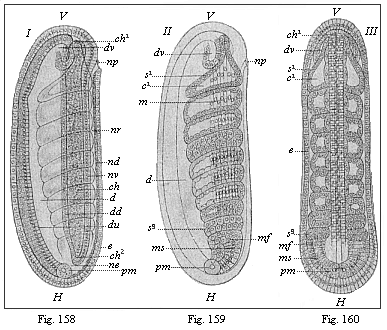
Figs. 158–160—Embryo of the amphioxus, twenty four hours old, with eight somites. (From Hatschek.) Figs. 158 and 159 lateral view (from left). Fig. 160 seen from back. In Fig. 158 only the outlines of the eight primitive segments are indicated, in Fig. 159 their cavities and muscular walls. V fore end, H hind end, d gut, du under and dd upper wall of the gut, ne canalis neurentericus, nv ventral, nd dorsal wall of the neural tube, np neuroporus, dv fore pouch of the gut, ch chorda, mf mesodermic fold, pm polar cells of the mesoderm (ms), e ectoderm.
In order to understand properly the real nature and origin of articulation in the human body and that of the higher vertebrates, it is necessary to compare it with that of the lower vertebrates, and bear in mind always the genetic connection of all the members of the stem. In this the simple development of the invaluable amphioxus once more furnishes the key to the complex and cenogenetically modified embryonic processes of the craniota. The articulation of the amphioxus begins at an early stage—earlier than in the craniotes. The two cœlom-pouches have hardly grown out of the primitive gut (Fig. 156 c) when the blind fore part of it (farthest away from the primitive mouth, u) begins to separate by a transverse fold (s): this is the first primitive segment. Immediately afterwards the hind part of the cœlom-pouches begins to divide into a series of pieces by new transverse folds (Fig. 157). The foremost of these primitive segments (us1) is the first and oldest; in Figs. 124 and 157 there are already five formed. They separate so rapidly, one behind the other, that eight pairs are formed within twenty-four hours of the beginning of development, and seventeen pairs twenty-four hours later. The number increases as the embryo grows and extends backwards, and new cells are formed constantly (at the primitive mouth) from the two primitive mesodermic cells (Figs. 159–160).
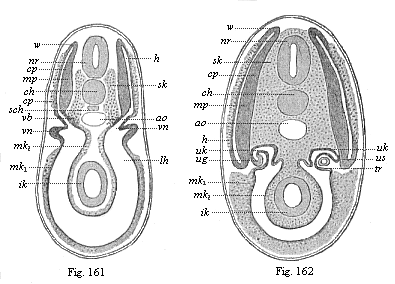
Figs. 161 and 162—Transverse section of shark-embryos (through the region of the kidneys). (From Wijhe and Hertwig.) In Fig. 162 the dorsal segment-cavities (h) are already separated from the body-cavity (lh), but they are connected a little earlier (Fig. 161), nr neural tube, ch chorda, sch subchordal string, ao aorta, sk skeletal-plate, mp muscle-plate, cp cutis-plate, w connection of latter (growth-zone), vn primitive kidneys, ug prorenal duct, uk prorenal canals, us point where they are cut off, tr prorenal funnel, mk middle germ-layer (mk1 parietal, mk2 visceral), ik inner germ-layer (gut-gland layer).
This typical articulation of the two cœlom-sacs begins very early in the lancelet, before they are yet severed from the primitive gut, so that at first each segment-cavity (us) still communicates by a narrow opening with the gut, like an intestinal gland. But this opening soon closes by complete severance, proceeding regularly backwards. The closed segments then extend more, so that their upper half grows upwards like a fold between the ectoderm (ak) and neural tube (n), and the lower half between the ectoderm and alimentary canal (ch; Fig. 82 d, left half of the figure). Afterwards the two halves completely separate, a lateral longitudinal fold cutting between them (mk, right half of Fig. 82). The dorsal segments (sd) provide the muscles of the trunk the whole length of the body (159): this cavity afterwards disappears. On the other hand, the ventral parts give rise, from their uppermost section, to the pronephridia or primitive-kidney canals, and from the lower to the segmental rudiments of the sexual glands or gonads. The partitions of the muscular dorsal pieces (myotomes) remain, and determine the permanent articulation of the vertebrate organism. But the partitions of the large ventral pieces (gonotomes) become thinner, and afterwards disappear in part, so that their cavities run together to form the metacœl, or the simple permanent body-cavity.
The articulation proceeds in substantially the same way in the other vertebrates, the craniota, starting from the cœlom-pouches. But whereas in the former case there is first a transverse division of the cœlom-sacs (by vertical folds) and then the dorso-ventral division, the procedure is reversed in the craniota; in their case each of the long cœlom-pouches first divides into a dorsal (primitive segment plates) and a ventral (lateral plates) section by a lateral longitudinal fold. Only the former are then broken up into primitive segments by the subsequent vertical folds; while the latter (segmented for a time in the amphioxus) remain undivided, and, by the divergence of their parietal and visceral plates, form a body-cavity that is unified from the first. In this case, again, it is clear that we must regard the features of the younger craniota as cenogenetically modified processes that can be traced palingenetically to the older acrania.
We have an interesting intermediate stage between the acrania and the fishes in these and many other respects in the cyclostoma (the hag and the lamprey, cf. Chapter XXI).
Fig. 163—Frontal (or horizontal-longitudinal) section of a triton-embryo with three pairs of primitive segments. ch chorda, us primitive segments, ush their cavity, ak horn plate.
Among the fishes the selachii, or primitive fishes, yield the most important information on these and many other phylogenetic questions (Figs. 161 and 162). The careful studies of Rückert, Van Wijhe, H. E. Ziegler, and others, have given us most valuable results. The products of the middle germinal layer are partly clear in these cases at the period when the dorsal primitive segment cavities (or myocœls, h) are still connected with the ventral body-cavity (lh; Fig. 161). In Fig. 162, a somewhat older embryo, these cavities are separated. The outer or lateral wall of the dorsal segment yields the cutis-plate (cp), the foundation of the connective corium. From its inner or median wall are developed the muscle-plate (mp, the rudiment of the trunk-muscles) and the skeletal plate, the formative matter of the vertebral column (sk).
In the amphibia, also, especially the water-salamander (Triton), we can observe very clearly the articulation of the cœlom-pouches and the rise of the primitive segments from their dorsal half (cf. Fig. 91, A, B, C). A horizontal longitudinal section of the salamander-embryo (Fig. 163) shows very clearly the series of pairs of these vesicular dorsal segments, which have been cut off on each side from the ventral side-plates, and lie to the right and left of the chorda.
Fig. 164—The third cervical vertebra
(human).
Fig. 165—The sixth dorsal vertebra (human).
Fig. 166—The second lumbar vertebra (human).
The metamerism of the amniotes agrees in all essential points with that of the three lower classes of vertebrates we have considered; but it varies considerably in detail, in consequence of cenogenetic disturbances that are due in the first place (like the degeneration of the cœlom-pouches) to the large development of the food-yelk. As the pressure of this seems to force the two middle layers together from the start, and as the solid structure of the mesoderm apparently belies the original hollow character of the sacs, the two sections of the mesoderm, which are at that time divided by the lateral fold—the dorsal segment-plates and ventral side-plates—have the appearance at first of solid layers of cells (Figs. 94–97). And when the articulation of the somites begins in the sole-shaped embryonic shield, and a couple of protovertebræ are developed in succession, constantly increasing in number towards the rear, these cube-shaped somites (formerly called protovertebræ, or primitive vertebræ) have the appearance of solid dice, made up of mesodermic cells (Fig. 93). Nevertheless, there is for a time a ventral cavity, or provertebral cavity, even in these solid “protovertebræ” (Fig. 143 uwh). This vesicular condition of the provertebra is of the greatest phylogenetic interest; we must, according to the cœlom theory, regard it as an hereditary reproduction of the hollow dorsal somites of the amphioxus (Figs. 156–160) and the lower vertebrates (Fig. 161–163). This rudimentary “provertebral cavity” has no physiological significance whatever in the amniote-embryo; it soon disappears, being filled up with cells of the muscular plate.
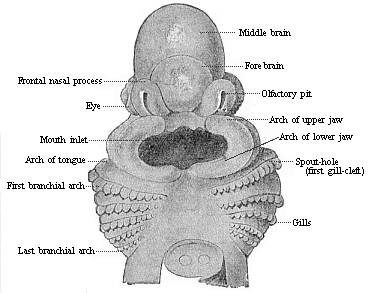
Fig. 167—Head of a shark embryo (Pristiurus), one-third of an inch long, magnified. (From Parker.) Seen from the ventral side.
The innermost median part of the primitive segment plates, which lies immediately on the chorda (Fig. 145 ch) and the medullary tube (m), forms the vertebral column in all the higher vertebrates (it is wanting in the lowest); hence it may be called the skeleton plate. In each of the provertebræ it is called the “sclerotome” (in opposition to the outlying muscular plate, the “myotome”). From the phylogenetic point of view the myotomes are much older than the sclerotomes. The lower or ventral part of each sclerotome (the inner and lower edge of the cube-shaped provertebra) divides into two plates, which grow round the chorda, and thus form the foundation of the body of the vertebra (wh). The upper plate presses between the chorda and the medullary tube, the lower between the chorda and the alimentary canal (Fig. 137 C). As the plates of two opposite provertebral pieces unite from the right and left, a circular sheath is formed round this part of the chorda. From this develops the body of a vertebra—that is to say, the massive lower or ventral half of the bony ring, which is called the “vertebra” proper and surrounds the medullary tube (Figs. 164–166). The upper or dorsal half of this bony ring, the vertebral arch (Fig. 145 wb), arises in just the same way from the upper part of the skeletal plate, and therefore from the inner and upper edge of the cube-shaped primitive vertebra. As the upper edges of two opposing somites grow together over the medullary tube from right and left, the vertebra-arch becomes closed.
The whole of the secondary vertebra, which is thus formed from the union of the skeletal plates of two provertebral pieces and encloses a part of the chorda in its body, consists at first of a rather soft mass of cells; this afterwards passes into a firmer, cartilaginous stage, and finally into a third, permanent, bony stage. These three stages can generally be distinguished in the greater part of the skeleton of the higher vertebrates; at first most parts of the skeleton are soft, tender, and membranous; they then become cartilaginous in the course of their development, and finally bony.
Fig. 168 and 169—Head of a chick embryo, of the third day. Fig. 168 from the front, Fig. 169 from the right. n rudimentary nose (olfactory pit), l rudimentary eye (optic pit, lens-cavity), g rudimentary ear (auditory pit), v fore-brain, gl eye-cleft. Of the three pairs of gill-arches the first has passed into a process of the upper jaw (o) and of the lower jaw (u). (From Kölliker.)
At the head part of the embryo in the amniotes there is not generally a cleavage of the middle germinal layer into provertebral and lateral plates, but the dorsal and ventral somites are blended from the first, and form what are called the “head-plates” (Fig. 148 k). From these are formed the skull, the bony case of the brain, and the muscles and corium of the body. The skull develops in the same way as the membranous vertebral column. The right and left halves of the head curve over the cerebral vesicle, enclose the foremost part of the chorda below, and thus finally form a simple, soft, membranous capsule about the brain. This is afterwards converted into a cartilaginous primitive skull, such as we find permanently in many of the fishes. Much later this cartilaginous skull becomes the permanent bony skull with its various parts. The bony skull in man and all the other amniotes is more highly differentiated and modified than that of the lower vertebrates, the amphibia and fishes. But as the one has arisen phylogenetically from the other, we must assume that in the former no less than the latter the skull was originally formed from the sclerotomes of a number of (at least nine) head-somites.
Fig. 170—Head of a dog embryo, seen from the front. a the two lateral halves of the foremost cerebral vesicle, b rudimentary eye, c middle cerebral vesicle, de first pair of gill-arches (e upper-jaw process, d lower-jaw process), f, f′, f″, second, third, and fourth pairs of gill-arches, g h i k heart (g right, h left auricle; i left, k right ventricle), l origin of the aorta with three pairs of arches, which go to the gill-arches. (From Bischoff.)
While the articulation of the vertebrate body is always obvious in the episoma or dorsal body, and is clearly expressed in the segmentation of the muscular plates and vertebræ, it is more latent in the hyposoma or ventral body. Nevertheless, the hyposomites of the vegetal half of the body are not less important than the episomites of the animal half. The segmentation in the ventral cavity affects the following principal systems of organs: 1, the gonads or sex-glands (gonotomes); 2, the nephridia or kidneys (nephrotomes); and 3, the head-gut with its gill-clefts (branchiotomes).
The metamerism of the hyposoma is less conspicuous because in all the craniotes the cavities of the ventral segments, in the walls of which the sexual products are developed, have long since coalesced, and formed a single large body-cavity, owing to the disappearance of the partition. This cenogenetic process is so old that the cavity seems to be unsegmented from the first in all the craniotes, and the rudiment of the gonads also is almost always unsegmented. It is the more interesting to learn that, according to the important discovery of Rückert, this sexual structure is at first segmental even in the actual selachii, and the several gonotomes only blend into a simple sexual gland on either side secondarily.
Amphioxus, the sole surviving representative of the acrania, once more yields us most interesting information; in this case the sexual glands remain segmented throughout life. The sexually mature lancelet has, on the right and left of the gut, a series of metamerous sacs, which are filled with ova in the female and sperm in the male. These segmental gonads are originally nothing else than the real gonotomes, separate body-cavities, formed from the hyposomites of the trunk.
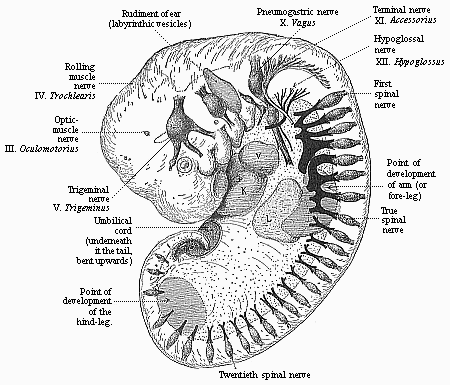
Fig. 171—Human embryo of the fourth week (twenty-six days old), one-fourth of an inch in length, magnified. (From Moll.) The rudiments of the cerebral nerves and the roots of the spinal nerves are especially marked. Underneath the four gill-arches (left side) is the heart (with auricle, V, and ventricle, K), under this again the liver (L).
The gonads are the most important segmental organs of the hyposoma, in the sense that they are phylogenetically the oldest. We find sexual glands (as pouch-like appendages of the gastro-canal system) in most of the lower animals, even in the medusæ, etc., which have no kidneys. The latter appear first (as a pair of excretory tubes) in the platodes (turbellaria), and have probably been inherited from these by the articulates (annelids) on the one hand and the unarticulated prochordonia on the other, and from these passed to the articulated vertebrates. The oldest form of the kidney system in this stem are the segmental pronephridia or prorenal canals, in the same arrangement as Boveri found them in the amphioxus. They are small canals that lie in the frontal plane, on each side of the chorda, between the episoma and hyposoma (Fig. 102 n); their internal funnel-shaped opening leads into the various body-cavities, their outer opening is the lateral furrow of the epidermis. Originally they must have had a double function, the carrying away of the urine from the episomites and the release of the sexual cells from the hyposomites.
The recent investigations of Ruckert and Van Wijhe on the mesodermic segments of the trunk and the excretory system of the selachii show that these “primitive fishes” are closely related to the amphioxus in this further respect. The transverse section of the shark-embryo in Fig. 161 shows this very clearly.
In other higher vertebrates, also, the kidneys develop (though very differently formed later on) from similar structures, which have been secondarily derived from the segmental pronephridia of the acrania. The parts of the mesoderm at which the first traces of them are found are usually called the middle or mesenteric plates. As the first traces of the gonads make their appearance in the lining of these middle plates nearer inward (or the middle) from the inner funnels of the nephro-canals, it is better to count this part of the mesoderm with the hyposoma.
The chief and oldest organ of the vertebrate hyposoma, the alimentary canal, is generally described as an unsegmented organ. But we could just as well say that it is the oldest of all the segmented organs of the vertebrate; the double row of the cœlom-pouches grows out of the dorsal wall of the gut, on either side of the chorda. In the brief period during which these segmental cœlom-pouches are still openly connected with the gut, they look just like a double chain of segmented visceral glands. But apart from this, we have originally in all vertebrates an important articulation of the fore-gut, that is wanting in the lower gut, the segmentation of the branchial (gill) gut.
Fig. 172—Transverse section of the shoulder and fore-limb (wing) of a chick-embryo of the fourth day, magnified about twenty times. Beside the medullary tube we can see on each side three clear streaks in the dark dorsal wall, which advance into the rudimentary fore-limb or wing (e). The uppermost of them is the muscular plate; the middle is the hind and the lowest the fore root of a spinal nerve. Under the chorda in the middle is the single aorta, at each side of it a cardinal vein, and below these the primitive kidneys. The gut is almost closed. The ventral wall advances into the amnion, which encloses the embryo. (From Remak.)
The gill-clefts, which originally in the older acrania pierced the wall of the fore-gut, and the gill-arches that separated them, were presumably also segmental, and distributed among the various metamera of the chain, like the gonads in the after-gut and the nephridia. In the amphioxus, too, they are still segmentally formed. Probably there was a division of labour of the hyposomites in the older (and long extinct) acrania, in such wise that those of the fore-gut took over the function of breathing and those of the after-gut that of reproduction. The former developed into gill-pouches, the latter into sex-pouches. There may have been primitive kidneys in both. Though the gills have lost their function in the higher animals, certain parts of them have been generally maintained in the embryo by a tenacious heredity. At a very early stage we notice in the embryo of man and the other amniotes, at each side of the head, the remarkable and important structures which we call the gill-arches and gill-clefts (Figs. 167–170 f). They belong to the characteristic and inalienable organs of the amniote-embryo, and are found always in the same spot and with the same arrangement and structure. There are formed to the right and left in the lateral wall of the fore-gut cavity, in its foremost part, first a pair and then several pairs of sac-shaped inlets, that pierce the whole thickness of the lateral wall of the head. They are thus converted into clefts, through which one can penetrate freely from without into the gullet. The wall thickens between these branchial folds, and changes into an arch-like or sickle-shaped piece—the gill, or gullet-arch. In this the muscles and skeletal parts of the branchial gut separate; a blood-vessel arch rises afterwards on their inner side (Fig. 98 ka). The number of the branchial arches and the clefts that alternate with them is four or five on each side in the higher vertebrates (Fig. 170 d, f, f′, f″). In some of the fishes (selachii) and in the cyclostoma we find six or seven of them permanently.
Fig. 173—Transverse section of the pelvic region and hind legs of a chick-embryo of the fourth day, magnified. h horn-plate, w medullary tube, n canal of the tube, u primitive kidneys, x chorda, e hind legs, b allantoic canal in the ventral wall, t aorta, v cardinal veins, a gut, d gut-gland layer, f gut-fibre layer, g embryonic epithelium, r dorsal muscles, c body-cavity or cœloma. (From Waldeyer.)
These remarkable structures had originally the function of respiratory organs—gills. In the fishes the water that serves for breathing, and is taken in at the mouth, still always passes out by the branchial clefts at the sides of the gullet. In the higher vertebrates they afterwards disappear. The branchial arches are converted partly into the jaws, partly into the bones of the tongue and the ear. From the first gill-cleft is formed the tympanic cavity of the ear.
There are few parts of the vertebrate organism that, like the outer covering or integument of the body, are not subject to metamerism. The outer skin (epidermis) is unsegmented from the first, and proceeds from the continuous horny plate. Moreover, the underlying cutis is also not metamerous, although it develops from the segmental structure of the cutis-plates (Figs. 161, 162 cp). The vertebrates are strikingly and profoundly different from the articulates in these respects also.
Further, most of the vertebrates still have a number of unarticulated organs, which have arisen locally, by adaptation of particular parts of the body to certain special functions. Of this character are the sense-organs in the episoma, and the limbs, the heart, the spleen, and the large visceral glands—lungs, liver, pancreas, etc.—in the hyposoma. The heart is originally only a local spindle-shaped enlargement of the large ventral blood-vessel or principal vein, at the point where the subintestinal passes into the branchial artery, at the limit of the head and trunk (Figs. 170, 171). The three higher sense-organs—nose, eye, and ear—were originally developed in the same form in all the craniotes, as three pairs of small depressions in the skin at the side of the head.
The organ of smell, the nose, has the appearance of a pair of small pits above the mouth-aperture, in front of the head (Fig. 169 n). The organ of sight, the eye, is found at the side of the head, also in the shape of a depression (Figs. 169 l, 170 b), to which corresponds a large outgrowth of the foremost cerebral vesicle on each side. Farther behind, at each side of the head, there is a third depression, the first trace of the organ of hearing (Fig. 169 g). As yet we can see nothing of the later elaborate structure of these organs, nor of the characteristic build of the face.
When the human embryo has reached When the human embryo has reached this stage of development, it can still scarcely be distinguished from that of any other higher vertebrate. All the chief parts of the body are now laid down: the head with the primitive skull, the rudiments of the three higher sense-organs and the five cerebral vesicles, and the gill-arches and clefts; the trunk with the spinal cord, the rudiment of the vertebral column, the chain of metamera, the heart and chief blood-vessels, and the kidneys. At this stage man is a higher vertebrate, but shows no essential morphological difference from the embryos of the mammals, the birds, the reptiles, etc. This is an ontogenetic fact of the utmost significance. From it we can gather the most important phylogenetic conclusions.
Fig. 174—Development of the lizard’s legs (Lacerta agilis), with special relation to their blood-vessels. 1, 3, 5, 7, 9, 11 right fore-leg; 13, 15 left fore-leg; 2, 4, 6, 8, 10, 12 right hind-leg; 14, 16 left hind-leg; SRV lateral veins of the trunk, VU umbilical vein. (From F. Hochstetter.)
There is still no trace of the limbs. Although head and trunk are separated and all the principal internal organs are laid down, there is no indication whatever of the “extremities” at this stage; they are formed later on. Here again we have a fact of the utmost interest. It proves that the older vertebrates had no feet, as we find to be the case in the lowest living vertebrates (amphioxus and the cyclostoma). The descendants of these ancient footless vertebrates only acquired extremities—two fore-legs and two hind-legs—at a much later stage of development. These were at first all alike, though they afterwards vary considerably in structure—becoming fins (of breast and belly) in the fishes, wings and legs in the birds, fore and hind legs in the creeping animals, arms and legs in the apes and man. All these parts develop from the same simple original structure, which forms secondarily from the trunk-wall (Figs. 172, 173). They have always the appearance of two pairs of small buds, which represent at first simple roundish knobs or plates. Gradually each of these plates becomes a large projection, in which we can distinguish a small inner part and a broader outer part. The latter is the rudiment of the foot or hand, the former that of the leg or arm. The similarity of the original rudiment of the limbs in different groups of vertebrates is very striking.
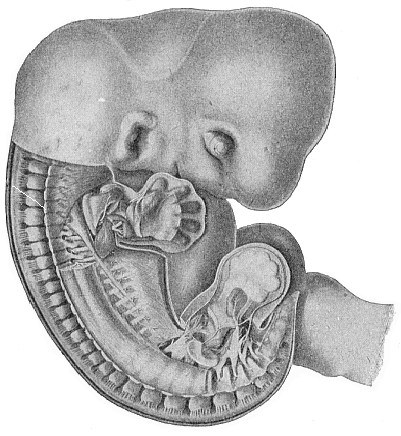
Fig. 175—Human embryo, five weeks old, half an inch long, seen from the right, magnified. (From Russel Bardeen and Harmon Lewis.) In the undissected head we see the eye, mouth, and ear. In the trunk the skin and part of the muscles have been removed, so that the cartilaginous vertebral column is free; the dorsal root of a spinal nerve goes out from each vertebra (towards the skin of the back). In the middle of the lower half of the figure part of the ribs and intercostal muscles are visible. The skin and muscles have also been removed from the right limbs; the internal rudiments of the five fingers of the hand, and five toes of the foot, are clearly seen within the fin-shaped plate, and also the strong network of nerves that goes from the spinal cord to the extremities. The tail projects under the foot, and to the right of it is the first part of the umbilical cord.
How the five fingers or toes with their blood-vessels gradually differentiate within the simple fin-like structure of the limbs can be seen in the instance of the lizard in Fig. 174. They are formed in just the same way in man: in the human embryo of five weeks the five fingers can clearly be distinguished within the fin-plate (Fig. 175).
The careful study and comparison of human embryos with those of other vertebrates at this stage of development is very instructive, and reveals more mysteries to the impartial student than all the religions in the world put together. For instance, if we compare attentively the three successive stages of development that are represented, in twenty different amniotes we find a remarkable likeness. When we see that as a fact twenty different amniotes of such divergent characters develop from the same embryonic form, we can easily understand that they may all descend from a common ancestor.
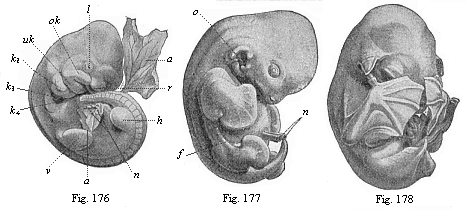
Figs. 176–178—Embryos of the bat (Vespertilio murinus) at three different stages. (From Oscar Schultze.) Fig. 176: Rudimentary limbs (v fore-leg, h hind-leg). l lenticular depression, r olfactory pit, ok upper jaw, uk lower jaw, k2, k3, k4 first, second and third gill-arches, a amnion, n umbilical vessel, d yelk-sac. Fig. 177: Rudiment of flying membrane, membranous fold between fore and hind leg. n umbilical vessel, o ear-opening, f flying membrane. Fig. 178: The flying membrane developed and stretched across the fingers of the hands, which cover the face.
In the first stage of development, in which the head with the five cerebral vesicles is already clearly indicated, but there are no limbs, the embryos of all the vertebrates, from the fish to man, are only incidentally or not at all different from each other. In the second stage, which shows the limbs, we begin to see differences between the embryos of the lower and higher vertebrates; but the human embryo is still hardly distinguishable from that of the higher mammals. In the third stage, in which the gill-arches have disappeared and the face is formed, the differences become more pronounced. These are facts of a significance that cannot be exaggerated.[27]
[27] Because they show how the most diverse structures may be developed from a common form. As we actually see this in the case of the embryos, we have a right to assume it in that of the stem-forms. Nevertheless, this resemblance, however great, is never a real identity. Even the embryos of the different individuals of one species are usually not really identical. If the reader can consult the complete edition of this work at a library, he will find six plates illustrating these twenty embryos.
If there is an intimate causal connection between the processes of embryology and stem-history, as we must assume in virtue of the laws of heredity, several important phylogenetic conclusions follow at once from these ontogenetic facts. The profound and remarkable similarity in the embryonic development of man and the other vertebrates can only be explained when we admit their descent from a common ancestor. As a fact, this common descent is now accepted by all competent scientists; they have substituted the natural evolution for the supernatural creation of organisms.
Among the many interesting phenomena that we have encountered in the course of human embryology, there is an especial importance in the fact that the development of the human body follows from the beginning just the same lines as that of the other viviparous mammals. As a fact, all the embryonic peculiarities that distinguish the mammals from other animals are found also in man; even the ovum with its distinctive membrane (zona pellucida, Fig. 14) shows the same typical structure in all mammals (apart from the older oviparous monotremes). It has long since been deduced from the structure of the developed man that his natural place in the animal kingdom is among the mammals. Linné (1735) placed him in this class with the apes, in one and the same order (primates), in his Systema Naturæ. This position is fully confirmed by comparative embryology. We see that man entirely resembles the higher mammals, and most of all the apes, in embryonic development as well as in anatomic structure. And if we seek to understand this ontogenetic agreement in the light of the biogenetic law, we find that it proves clearly and necessarily the descent of man from a series of other mammals, and proximately from the primates. The common origin of man and the other mammals from a single ancient stem-form can no longer be questioned; nor can the immediate blood-relationship of man and the ape.
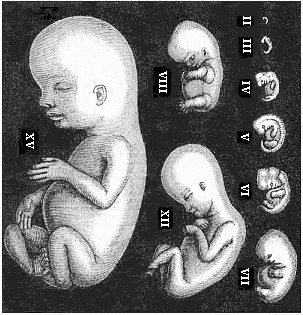
Fig. 179—Human embryos from the second to the fifteenth week, seen from the left, the curved back turned towards the right. (Mostly from Ecker.) II of fourteen days. III of three weeks. IV of four weeks. V of five weeks. VI of six weeks. VII of seven weeks. VIII of eight weeks. XII of twelve weeks. XV of fifteen weeks.
The essential agreement in the whole bodily form and inner structure is still visible in the embryo of man and the other mammals at the late stage of development at which the mammal-body can be recognised as such. But at a somewhat earlier stage, in which the limbs, gill-arches, sense-organs, etc., are already outlined, we cannot yet recognise the mammal embryos as such, or distinguish them from those of birds and reptiles. When we consider still earlier stages of development, we are unable to discover any essential difference in bodily structure between the embryos of these higher vertebrates and those of the lower, the amphibia and fishes. If, in fine, we go back to the construction of the body out of the four germinal layers, we are astonished to perceive that these four layers are the same in all vertebrates, and everywhere take a similar part in the building-up of the fundamental organs of the body. If we inquire as to the origin of these four secondary layers, we learn that they always arise in the same way from the two primary layers; and the latter have the same significance in all the metazoa (i.e., all animals except the unicellulars). Finally, we see that the cells which make up the primary germinal layers owe their origin in every case to the repeated cleavage of a single simple cell, the stem-cell or fertilised ovum.
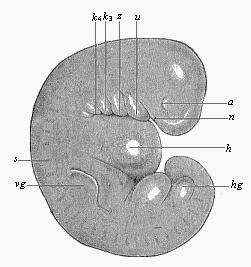
Fig. 180—Very young human embryo of the fourth week, one-fourth of an inch long (taken from the womb of a suicide eight hours after death). (From Rabl.) n nasal pits, a eye, u lower jaw, z arch of hyoid bone, k3 and k4 third and fourth gill-arch, h heart; s primitive segments, vg fore-limb (arm), hg hind-limb (leg), between the two the ventral pedicle.
It is impossible to lay too much stress on this remarkable agreement in the chief embryonic features in man and the other animals. We shall make use of it later on for our monophyletic theory of descent—the hypothesis of a common descent of man and all the metazoa from the gastræa. The first rudiments of the principal parts of the body, especially the oldest organ, the alimentary canal, are the same everywhere; they have always the same extremely simple form. All the peculiarities that distinguish the various groups of animals from each other only appear gradually in the course of embryonic development; and the closer the relation of the various groups, the later they are found. We may formulate this phenomenon in a definite law, which may in a sense be regarded as an appendix to our biogenetic law. This is the law of the ontogenetic connection of related animal forms. It runs: The closer the relation of two fully-developed animals in respect of their whole bodily structure, and the nearer they are connected in the classification of the animal kingdom, the longer do their embryonic forms retain their identity, and the longer is it impossible (or only possible on the ground of subordinate features) to distinguish between their embryos. This law applies to all animals whose embryonic development is, in the main, an hereditary summary of their ancestral history, or in which the original form of development has been faithfully preserved by heredity. When, on the other hand, it has been altered by cenogenesis, or disturbance of development, we find a limitation of the law, which increases in proportion to the introduction of new features by adaptation (cf. Chapter I, pp. 4–6). Thus the apparent exceptions to the law can always be traced to cenogenesis.
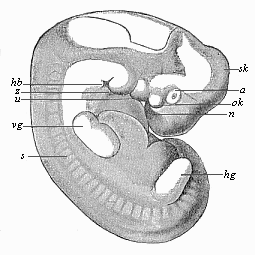
Fig. 181—Human embryo of the middle of the fifth week, one-third of an inch long. (From Rabl.) Letters as in Fig. 180, except sk curve of skull, ok upper jaw, hb neck-indentation.
When we apply to man this law of the ontogenetic connection of related forms, and run rapidly over the earliest stages of human development with an eye to it, we notice first of all the structural identity of the ovum in man and the other mammals at the very beginning (Figs. 1, 14). The human ovum possesses all the distinctive features of the ovum of the viviparous mammals, especially the characteristic formation of its membrane (zona pellucida), which clearly distinguishes it from the ovum of all other animals. When the human fœtus has attained the age of fourteen days, it forms a round vesicle (or “embryonic vesicle”) about a quarter of an inch in diameter. A thicker part of its border forms a simple sole-shaped embryonic shield one-twelfth of an inch long (Fig. 133). On its dorsal side we find in the middle line the straight medullary furrow, bordered by the two parallel dorsal or medullary swellings. Behind, it passes by the neurenteric canal into the primitive gut or primitive groove. From this the folding of the two cœlom-pouches proceeds in the same way as in the other mammals (cf. Fig. 96, 97). In the middle of the sole-shaped embryonic shield the first primitive segments immediately begin to make their appearance. At this age the human embryo cannot be distinguished from that of other mammals, such as the hare or dog.
A week later (or after the twenty-first day) the human embryo has doubled its length; it is now about one-fifth of an inch long, and, when seen from the side, shows the characteristic bend of the back, the swelling of the head-end, the first outline of the three higher sense-organs, and the rudiments of the gill-clefts, which pierce the sides of the neck (Fig. 179, III). The allantois has grown out of the gut behind. The embryo is already entirely enclosed in the amnion, and is only connected in the middle of the belly by the vitelline duct with the embryonic vesicle, which changes into the yelk-sac. There are no extremities or limbs at this stage, no trace of arms or legs. The head-end has been strongly differentiated from the tail-end; and the first outlines of the cerebral vesicles in front, and the heart below, under the fore-arm, are already more or less clearly seen. There is as yet no real face. Moreover, we seek in vain at this stage a special character that may distinguish the human embryo from that of other mammals.
A week later (after the fourth week, on the twenty-eighth to thirtieth day of development) the human embryo has reached a length of about one-third of an inch (Fig 179 IV). We can now clearly distinguish the head with its various parts; inside it the five primitive cerebral vesicles (fore-brain, middle-brain, intermediate-brain, hind-brain, and after-brain); under the head the gill-arches, which divide the gill-clefts; at the sides of the head the rudiments of the eyes, a couple of pits in the outer skin, with a pair of corresponding simple vesicles growing out of the lateral wall of the fore-brain (Figs. 180, 181 a). Far behind the eyes, over the last gill-arches, we see a vesicular rudiment of the auscultory organ. The rudimentary limbs are now clearly outlined—four simple buds of the shape of round plates, a pair of fore (vg) and a pair of hind legs (hg), the former a little larger than the latter. The large head bends over the trunk, almost at a right angle. The latter is still connected in the middle of its ventral side with the embryonic vesicle; but the embryo has still further severed itself from it, so that it already hangs out as the yelk-sac. The hind part of the body is also very much curved, so that the pointed tail-end is directed towards the head. The head and face-part are sunk entirely on the still open breast. The bend soon increases so much that the tail almost touches the forehead (Fig. 179 V.; Fig. 181). We may then distinguish three or four special curves on the round dorsal surface—namely, a skull-curve in the region of the second cerebral vesicle, a neck-curve at the beginning of the spinal cord, and a tail-curve at the fore-end. This pronounced curve is only shared by man and the higher classes of vertebrates (the amniotes); it is much slighter, or not found at all, in the lower vertebrates. At this age (four weeks) man has a considerable tail, twice as long as his legs. A vertical longitudinal section through the middle plane of this tail (Fig. 182) shows that the hinder end of the spinal marrow extends to the point of the tail, as also does the underlying chorda (ch), the terminal continuation of the vertebral column. Of the latter, the rudiments of the seven coccygeal (or lowest) vertebræ are visible—thirty-two indicates the third and thirty-six the seventh of these. Under the vertebral column we see the hindmost ends of the two large blood-vessels of the tail, the principal artery (aorta caudalis or arteria sacralis media, Ao), and the principal vein (vena caudalis or sacralis media). Underneath is the opening of the anus (an) and the urogenital sinus (S.ug). From this anatomic structure of the human tail it is perfectly clear that it is the rudiment of an ape-tail, the last hereditary relic of a long hairy tail, which has been handed down from our tertiary primate ancestors to the present day.
Fig. 182—Median longitudinal section of the tail of a human embryo, two-thirds of an inch long. (From Ross Granville Harrison.) Med medullary tube, Ca.fil caudal filament, ch chorda, ao caudal artery, V.c.i caudal vein, an anus, S.ug sinus urogenitalis.
Fig. 183—Human embryo, four weeks old, opened on the ventral side. Ventral and dorsal walls are cut away, so as to show the contents of the pectoral and abdominal cavities. All the appendages are also removed (amnion, allantois, yelk-sac), and the middle part of the gut. n eye, 3 nose, 4 upper jaw, 5 lower jaw, 6 second, 6″ third gill-arch, ov heart (o right, o′ left auricle; v right, v′ left ventricle), b origin of the aorta, f liver (u umbilical vein), e gut (with vitelline artery, cut off at a′), j′ vitelline vein, m primitive kidneys, t rudimentary sexual glands, r terminal gut (cut off at the mesentery z), n umbilical artery, u umbilical vein, 9 fore-leg, 9′ hind-leg. (From Coste.)
Fig. 184—Human embryo, five weeks old, opened from the ventral side (as in Fig. 183). Breast and belly-wall and liver are removed. 3 outer nasal process, 4 upper jaw, 5 lower jaw, z tongue, v right, v′ left ventricle of heart, o′ left auricle, b origin of aorta, b′, b″, b‴ first, second, and third aorta-arches, c, c′, c″ vena cava, ae lungs (y pulmonary artery), e stomach, m primitive kidneys (j left vitelline vein, s cystic vein, a right vitelline artery, n umbilical artery, u umbilical vein), x vitelline duct, i rectum, 8 tail, 9 fore-leg, 9′ hind-leg. (From Coste.)
It sometimes happens that we find even external relics of this tail growing. According to the illustrated works of Surgeon-General Bernhard Ornstein, of Greece, these tailed men are not uncommon; it is not impossible that they gave rise to the ancient fables of the satyrs. A great number of such cases are given by Max Bartels in his essay on “Tailed Men” (1884, in the Archiv für Anthropologie, Band XV), and critically examined. These atavistic human tails are often mobile; sometimes they contain only muscles and fat, sometimes also rudiments of caudal vertebræ. They have a length of eight to ten inches and more. Granville Harrison has very carefully studied one of these cases of “pigtail,” which he removed by operation from a six months old child in 1901. The tail moved briskly when the child cried or was excited, and was drawn up when at rest.
Fig. 186—Human ovum of twelve to thirteen days (?). (From Allen Thomson.) 1. Not opened. 2. Opened and magnified. Within the outer chorion the tiny curved fœtus lies on the large embryonic vesicle, to the left above.
Fig. 187—Human ovum of ten days. (From
Allen Thomson.) Opened; the small fœtus in the right half, above.
Fig. 188—Human fœtus of ten days, taken
from the preceding ovum, magnified, a yelk-sac, b neck (the
medullary groove already closed), c head (with open medullary groove),
d hind part (with open medullary groove), e a shred of the
amnion.
Fig. 189—Human ovum of twenty to twenty-two
days. (From Allen Thomson.) Opened. The chorion forms a spacious
vesicle, to the inner wall of which the small fœtus (to the right above) is
attached by a short umbilical cord.
Fig. 190—Human fœtus of twenty to
twenty-two days, taken from the preceding ovum, magnified. a amnion,
b yelk-sac, c lower-jaw process of the first gill-arch, d
upper-jaw process of same, e second gill-arch (two smaller ones behind).
Three gill-clefts are clearly seen. f rudimentary fore-leg, g
auditory vesicle, h eye, i heart.
In the opinion of some travellers and anthropologists, the atavistic tail-formation is hereditary in certain isolated tribes (especially in south-eastern Asia and the archipelago), so that we might speak of a special race or “species” of tailed men (Homo caudatus). Bartels has “no doubt that these tailed men will be discovered in the advance of our geographical and ethnographical knowledge of the lands in question” (Archiv für Anthropologie, Band XV, p. 129).
Fig. 191—Human embryo of sixteen to eighteen days. (From Coste.) Magnified. The embryo is surrounded by the amnion, (a), and lies free with this in the opened embryonic vesicle. The belly is drawn up by the large yelk-sac (d), and fastened to the inner wall of the embryonic membrane by the short and thick pedicle (b). Hence the normal convex curve of the back (Fig. 190) is here changed into an abnormal concave surface. h heart, m parietal mesoderm. The spots on the outer wall of the serolemma are the roots of the branching chorion-villi, which are free at the border.
When we open a human embryo of one month (Fig. 183), we find the alimentary canal formed in the body-cavity, and for the most part cut off from the embryonic vesicle. There are both mouth and anus apertures. But the mouth-cavity is not yet separated from the nasal cavity, and the face not yet shaped. The heart shows all its four sections; it is very large, and almost fills the whole of the pectoral cavity (Fig. 183 ov). Behind it are the very small rudimentary lungs. The primitive kidneys (m) are very large; they fill the greater part of the abdominal cavity, and extend from the liver (f) to the pelvic gut. Thus at the end of the first month all the chief organs are already outlined. But there are at this stage no features by which the human embryo materially differs from that of the dog, the hare, the ox, or the horse—in a word, of any other higher mammal. All these embryos have the same, or at least a very similar, form; they can at the most be distinguished from the human embryo by the total size of the body or some other insignificant difference in size. Thus, for instance, in man the head is larger in proportion to the trunk than in the ox. The tail is rather longer in the dog than in man. These are all negligible differences. On the other hand, the whole internal organisation and the form and arrangement of the various organs are essentially the same in the human embryo of four weeks as in the embryos of the other mammals at corresponding stages.
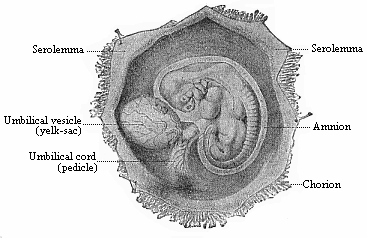
Fig. 192—Human embryo of the fourth week, one-third of an inch long, lying in the dissected chorion.
Fig. 193—Human embryo of the fourth week, with its membranes, like Fig. 192, but a little older. The yelk-sac is rather smaller, the amnion and chorion larger.
It is otherwise in the second month of human development. Fig. 179 represents a human embryo of six weeks (VI), one of seven weeks (VII), and one of eight weeks (VIII), at natural size. The differences which mark off the human embryo from that of the dog and the lower mammals now begin to be more pronounced. We can see important differences at the sixth, and still more at the eighth week, especially in the formation of the head. The size of the various sections of the brain is greater in man, and the tail is shorter.
Other differences between man and the lower mammals are found in the relative size of the internal organs. But even at this stage the human embryo differs very little from that of the nearest related mammals—the apes, especially the anthropomorphic apes.
The features by means of which we distinguish between them are not clear until later on. Even at a much more advanced stage of development, when we can distinguish the human fœtus from that of the ungulates at a glance, it still closely resembles that of the higher apes. At last we get the distinctive features, and we can distinguish the human embryo confidently at the first glance from that of all other mammals during the last four months of fœtal life—from the sixth to the ninth month of pregnancy. Then we begin to find also the differences between the various races of men, especially in regard to the formation of the skull and the face. (Cf. Chapter XXIII.)
Fig. 194—Human embryo with its membranes, six weeks old. The outer envelope of the whole ovum is the chorion, thickly covered with its branching villi, a product of the serous membrane. The embryo is enclosed in the delicate amnion-sac. The yelk-sac is reduced to a small pear-shaped umbilical vesicle; its thin pedicle, the long vitelline duct, is enclosed in the umbilical cord. In the latter, behind the vitelline duct, is the much shorter pedicle of the allantois, the inner lamina of which (the gut-gland layer) forms a large vesicle in most of the mammals, while the outer lamina is attached to the inner wall of the outer embryonic coat, and forms the placenta there. (Half diagrammatic.)
The striking resemblance that persists so long between the embryo of man and of the higher apes disappears much earlier in the lower apes. It naturally remains longest in the large anthropomorphic apes (gorilla, chimpanzee, orang, and gibbon). The physiognomic similarity of these animals, which we find so great in their earlier years, lessens with the increase of age. On the other hand, it remains throughout life in the remarkable long-nosed ape of Borneo (Nasalis larvatus). Its finely-shaped nose would be regarded with envy by many a man who has too little of that organ. If we compare the face of the long-nosed ape with that of abnormally ape-like human beings (such as the famous Miss Julia Pastrana, Fig. 185), it will be admitted to represent a higher stage of development. There are still people among us who look especially to the face for the “image of God in man.” The long-nosed ape would have more claim to this than some of the stumpy-nosed human individuals one meets.
This progressive divergence of the human from the animal form, which is based on the law of the ontogenetic connection between related forms, is found in the structure of the internal organs as well as in external form. It is also expressed in the construction of the envelopes and appendages that we find surrounding the fœtus externally, and that we will now consider more closely. Two of these appendages—the amnion and the allantois—are only found in the three higher classes of vertebrates, while the third, the yelk-sac, is found in most of the vertebrates. This is a circumstance of great importance, and it gives us valuable data for constructing man’s genealogical tree.
As regards the external membrane that encloses the ovum in the mammal womb, we find it just the same in man as in the higher mammals. The ovum is, the reader will remember, first surrounded by the transparent structureless ovolemma or zona pellucida (Figs. 1, 14). But very soon, even in the first week of development, this is replaced by the permanent chorion. This is formed from the external layer of the amnion, the serolemma, or “serous membrane,” the formation of which we shall consider presently; it surrounds the fœtus and its appendages as a broad, completely closed sac; the space between the two, filled with clear watery fluid, is the serocœlom, or interamniotic cavity (“extra-embryonic body-cavity”). But the smooth surface of the sac is quickly covered with numbers of tiny tufts, which are really hollow outgrowths like the fingers of a glove (Figs. 186, 191, 198 chz). They ramify and push into the corresponding depressions that are formed by the tubular glands of the mucous membrane of the maternal womb. Thus, the ovum secures its permanent seat (Fig. 186–194).
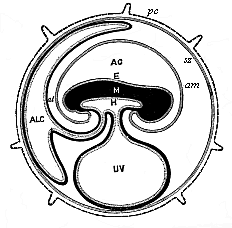
Fig. 195—Diagram of the embryonic organs of the mammal (fœtal membranes and appendages). (From Turner.) E, M, H outer, middle, and inner germ layer of the embryonic shield, which is figured in median longitudinal section, seen from the left. am amnion. AC amniotic cavity, UV yelk-sac or umbilical vesicle, ALC allantois, al pericœlom or serocœlom (inter-amniotic cavity), sz serolemma (or serous membrane), pc prochorion (with villi).)
In human ova of eight to twelve days this external membrane, the chorion, is already covered with small tufts or villi, and forms a ball or spheroid of one-fourth to one-third of an inch in diameter (Figs. 186–188). As a large quantity of fluid gathers inside it, the chorion expands more and more, so that the embryo only occupies a small part of the space within the vesicle. The villi of the chorion grow larger and more numerous. They branch out more and more. At first the villi cover the whole surface, but they afterwards disappear from the greater part of it; they then develop with proportionately greater vigour at a spot where the placenta is formed from the allantois.
When we open the chorion of a human embryo of three weeks, we find on the ventral side of the fœtus a large round sac, filled with fluid. This is the yelk-sac, or “umbilical vesicle,” the origin of which we have considered previously. The larger the embryo becomes the smaller we find the yelk-sac. In the end we find the remainder of it in the shape of a small pear-shaped vesicle, fastened to a long thin stalk (or pedicle), and hanging from the open belly of the fœtus (Fig. 194). This pedicle is the vitelline duct, and is separated from the body at the closing of the navel.
Behind the yelk-sac a second appendage, of much greater importance, is formed at an early stage at the belly of the mammal embryo. This is the allantois or “primitive urinary sac,” an important embryonic organ, only found in the three higher classes of vertebrates. In all the amniotes the allantois quickly appears at the hinder end of the alimentary canal, growing out of the cavity of the pelvic gut (Fig. 147 r, u, Fig. 195 ALC).
The further development of the allantois varies considerably in the three sub-classes of the mammals. The two lower sub-classes, monotremes and marsupials, retain the simpler structure of their ancestors, the reptiles. The wall of the allantois and the enveloping serolemma remains smooth and without villi, as in the birds. But in the third sub-class of the mammals the serolemma forms, by invagination at its outer surface, a number of hollow tufts or villi, from which it takes the name of the chorion or mallochorion. The gut-fibre layer of the allantois, richly supplied with branches of the umbilical vessel, presses into these tufts of the primary chorion, and forms the “secondary chorion.” Its embryonic blood-vessels are closely correlated to the contiguous maternal blood-vessels of the environing womb, and thus is formed the important nutritive apparatus of the embryo which we call the placenta.
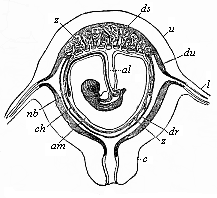
Fig. 196—Diagrammatic frontal section of the pregnant human womb. (From Longet.) The embryo hangs by the umbilical cord, which encloses the pedicle of the allantois (al). nb umbilical vessel, am amnion, ch chorion, ds decidua serotina, dv decidua vera, dr decidua reflexa, z villi of the placenta, c cervix uteri, u uterus.)
The pedicle of the allantois, which connects the embryo with the placenta and conducts the strong umbilical vessels from the former to the latter, is covered by the amnion, and, with this amniotic sheath and the pedicle of the yelk-sac, forms what is called the umbilical cord (Fig. 196 al). As the large and blood-filled vascular network of the fœtal allantois attaches itself closely to the mucous lining of the maternal womb, and the partition between the blood-vessels of mother and child becomes much thinner, we get that remarkable nutritive apparatus of the fœtal body which is characteristic of the placentalia (or choriata). We shall return afterwards to the closer consideration of this (cf. Chapter XXIII).
In the various orders of mammals the placenta undergoes many modifications, and these are in part of great evolutionary importance and useful in classification. There is only one of these that need be specially mentioned—the important fact, established by Selenka in 1890, that the distinctive human placentation is confined to the anthropoids. In this most advanced group of the mammals the allantois is very small, soon loses its cavity, and then, in common with the amnion, undergoes certain peculiar changes. The umbilical cord develops in this case from what is called the “ventral pedicle.” Until very recently this was regarded as a structure peculiar to man. We now know from Selenka that the much-discussed ventral pedicle is merely the pedicle of the allantois, combined with the pedicle of the amnion and the rudimentary pedicle of the yelk-sac. It has just the same structure in the orang and gibbon (Fig. 197) and very probably in the chimpanzee and gorilla, as in man; it is, therefore, not a disproof, but a striking fresh proof, of the blood-relationship of man and the anthropoid apes.
We find only in the anthropoid apes—the gibbon and orang of Asia and the chimpanzee and gorilla of Africa—the peculiar and elaborate formation of the placenta that characterises man (Fig. 198). In this case there is at an early stage an intimate blending of the chorion of the embryo and the part of the mucous lining of the womb to which it attaches. The villi of the chorion with the blood-vessels they contain grow so completely into the tissue of the uterus, which is rich in blood, that it becomes impossible to separate them, and they form together a sort of cake. This comes away as the “afterbirth” at parturition; at the same time, the part of the mucous lining of the womb that has united inseparably with the chorion is torn away; hence it is called the decidua (“falling-away membrane”), and also the “sieve-membrane,” because it is perforated like a sieve. We find a decidua of this kind in most of the higher placentals; but it is only in man and the anthropoid apes that it divides into three parts—the outer, inner, and placental decidua. The external or true decidua (Fig. 196 du, Fig. 199 g) is the part of the mucous lining of the womb that clothes the inner surface of the uterine cavity wherever it is not connected with the placenta. The placental or spongy decidua (placentalis or serotina, Fig. 196 ds, Fig. 199 d) is really the placenta itself, or the maternal part of it (placenta uterina)—namely, that part of the mucous lining of the womb which unites intimately with the chorion-villi of the fœtal placenta. The internal or false decidua (interna or reflexa, Fig. 196 dr, Fig. 199 f) is that part of the mucous lining of the womb which encloses the remaining surface of the ovum, the smooth chorion (chorion læve), in the shape of a special thin membrane. The origin of these three different deciduous membranes, in regard to which quite erroneous views (still retained in their names) formerly prevailed, is now quite clear, The external decidua vera is the specially modified and subsequently detachable superficial stratum of the original mucous lining of the womb. The placental decidua serotina is that part of the preceding which is completely transformed by the ingrowth of the chorion-villi, and is used for constructing the placenta. The inner decidua reflexa is formed by the rise of a circular fold of the mucous lining (at the border of the decidua vera and serotina), which grows over the fœtus (like the anmnion) to the end.
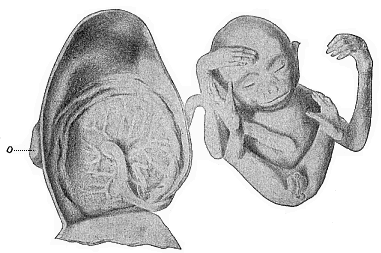
Fig. 197—Male embryo of the Siamang-gibbon (Hylobates siamanga) of Sumatra; to the left the dissected uterus, of which only the dorsal half is given. The embryo has been taken out, and the limbs folded together; it is still connected by the umbilical cord with the centre of the circular placenta which is attached to the inside of the womb. This embryo takes the head-position in the womb, and this is normal in man also.
The peculiar anatomic features that characterise the human fœtal membranes are found in just the same way in the higher apes. Until recently it was thought that the human embryo was distinguished by its peculiar construction of a solid allantois and a special ventral pedicle, and that the umbilical cord developed from this in a different way than in the other mammals. The opponents of the unwelcome “ape-theory” laid great stress on this, and thought they had at last discovered an important indication that separated man from all the other placentals. But the remarkable discoveries published by the distinguished zoologist Selenka in 1890 proved that man shares these peculiarities of placentation with the anthropoid apes, though they are not found in the other apes. Thus the very feature which was advanced by our critics as a disproof became a most important piece of evidence in favour of our pithecoid origin.)
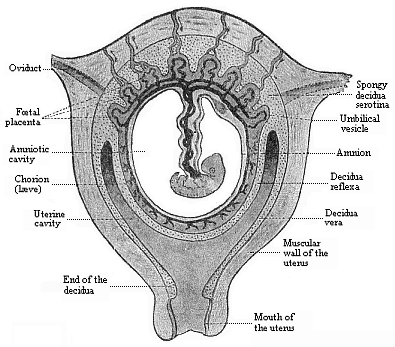
Fig. 198—Frontal section of the pregnant human womb. (From Turner.) The embryo (a month old) hangs in the middle of the amniotic cavity by the ventral pedicle or umbilical cord, which connects it with the placenta (above).
Of the three vesicular appendages of the amniote embryo which we have now described the amnion has no blood-vessels at any moment of its existence. But the other two vesicles, the yelk-sac and the allantois, are equipped with large blood-vessels, and these effect the nourishment of the embryonic body. We may take the opportunity to make a few general observations on the first circulation in the embryo and its central organ, the heart. The first blood-vessels, the heart, and the first blood itself, are formed from the gut-fibre layer. Hence it was called by earlier embryologists the “vascular layer.” In a sense the term is quite correct. But it must not be understood as if all the blood-vessels in the body came from this layer, or as if the whole of this layer were taken up only with the formation of blood-vessels. Neither of these suppositions is true. Blood-vessels may be formed independently in other parts, especially in the various products of the skin-fibre layer.
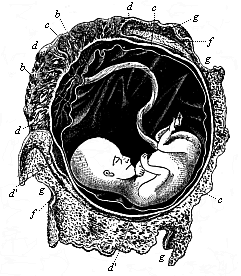
Fig. 199—Human fœtus, twelve weeks old, with its membranes. The umbilical cord goes from its navel to the placenta. b amnion, c chorion, d placenta, d apostrophe, relics of villi on smooth chorion, f internal or reflex decidua, g external or true decidua. (From B. Schultze.)
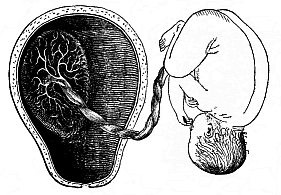
Fig. 200—Mature human fœtus (at the end of pregnancy, in its natural position, taken out of the uterine cavity). On the inner surface of the latter (to the left) is the placenta, which is connected by the umbilical cord with the child’s navel. (From Bernhard Schultze.)
The first blood-vessels of the mammal embryo have been considered by us previously, and we shall study the development of the heart in the second volume.
In every vertebrate it lies at first in the ventral wall of the fore-gut, or in the ventral (or cardiac) mesentery, by which it is connected for a time with the wall of the body. But it soon severs itself from the place of its origin, and lies freely in a cavity—the cardiac cavity. For a short time it is still connected with the former by the thin plate of the mesocardium. Afterwards it lies quite free in the cardiac cavity, and is only directly connected with the gut-wall by the vessels which issue from it.
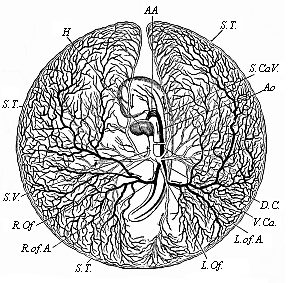
Fig. 201—Vitelline vessels in the germinative area of a chick-embryo, at the close of the third day of incubation. (From Balfour.) The detached germinative area is seen from the ventral side: the arteries are dark, the veins light. H heart, AA aorta-arches, Ao aorta, R.of.A right omphalo-mesenteric artery, S.T. sinus terminalis, L.Of and R.Of right and left omphalo-mesenteric veins, S.V. sinus venosus, D.C. ductus Cuvieri, S.Ca.V. and V.Ca. fore and hind cardinal veins.
The fore-end of the spindle-shaped tube, which soon bends into an S-shape (Figure 1.202), divides into a right and left branch. These tubes are bent upwards arch-wise, and represent the first arches of the aorta. They rise in the wall of the fore-gut, which they enclose in a sense, and then unite above, in the upper wall of the fore gut-cavity, to form a large single artery, that runs backward immediately under the chorda, and is called the aorta (Fig. 201 Ao). The first pair of aorta-arches rise on the inner wall of the first pair of gill-arches, and so lie between the first gill-arch (k) and the fore-gut (d), just as we find them throughout life in the fishes. The single aorta, which results from the conjunction of these two first vascular arches, divides again immediately into two parallel branches, which run backwards on either side of the chorda. These are the primitive aortas which we have already mentioned; they are also called the posterior vertebral arteries. These two arteries now give off at each side, behind, at right angles, four or five branches, and these pass from the embryonic body to the germinative area, they are called omphalo-mesenteric or vitelline arteries. They represent the first beginning of a fœtal circulation. Thus, the first blood-vessels pass over the embryonic body and reach as far as the edge of the germinative area. At first they are confined to the dark or “vascular” area. But they afterwards extend over the whole surface of the embryonic vesicle. In the end, the whole of the yelk-sac is covered with a vascular net-work. These vessels have to gather food from the contents of the yelk-sac and convey it to the embryonic body. This is done by the veins, which pass first from the germinative area, and afterwards from the yelk-sac, to the farther end of the heart. They are called vitelline, or, frequently, omphalo-mesenteric, veins.
These vessels naturally atrophy with the degeneration of the umbilical vesicle, and the vitelline circulation is replaced by a second, that of the allantois. Large blood-vessels are developed in the wall of the urinary sac or the allantois, as before, from the gut-fibre layer. These vessels grow larger and larger, and are very closely connected with the vessels that develop in the body of the embryo itself. Thus, the secondary, allantoic circulation gradually takes the place of the original vitelline circulation. When the allantois has attached itself to the inner wall of the chorion and been converted into the placenta, its blood-vessels alone effect the nourishment of the embryo. They are called umbilical vessels, and are originally double—a pair of umbilical arteries and a pair of umbilical veins. The two umbilical veins (Fig. 183 u), which convey blood from the placenta to the heart, open it first into the united vitelline veins. The latter then disappear, and the right umbilical vein goes with them, so that henceforth a single large vein, the left umbilical vein, conducts all the blood from the placenta to the heart of the embryo. The two arteries of the allantois, or the umbilical arteries (Figs. 183 n, 184 n), are merely the ultimate terminations of the primitive aortas, which are strongly developed afterwards. This umbilical circulation is retained until the nine months of embryonic life are over, and the human embryo enters into the world as the independent individual. The umbilical cord (Fig. 196 al), in which these large blood-vessels pass from the embryo to the placenta, comes away, together with the latter, in the after-birth, and with the use of the lungs begins an entirely new form of circulation, which is confined to the body of the infant.
Fig. 202—Boat-shaped embryo of the dog, from the ventral side, magnified. In front under the forehead we can see the first pair of gill-arches; underneath is the S-shaped heart, at the sides of which are the auditory vesicles. The heart divides behind into the two vitelline veins, which expand in the germinative area (which is torn off all round). On the floor of the open belly lie, between the protovertebræ, the primitive aortas, from which five pairs of vitelline arteries are given off. (From Bischoff.)
There is a great phylogenetic significance in the perfect agreement which we find between man and the anthropoid apes in these important features of embryonic circulation, and the special construction of the placenta and the umbilical cord. We must infer from it a close blood-relationship of man and the anthropomorphic apes—a common descent of them from one and the same extinct group of lower apes. Huxley’s “pithecometra-principle” applies to these ontogenetic features as much as to any other morphological relations: “The differences in construction of any part of the body are less between man and the anthropoid apes than between the latter and the lower apes.”
This important Huxleian law, the chief consequence of which is “the descent of man from the ape,” has lately been confirmed in an interesting and unexpected way from the side of the experimental physiology of the blood. The experiments of Hans Friedenthal at Berlin have shown that human blood, mixed with the blood of lower apes, has a poisonous effect on the latter; the serum of the one destroys the blood-cells of the other. But this does not happen when human blood is mixed with that of the anthropoid ape. As we know from many other experiments that the mixture of two different kinds of blood is only possible without injury in the case of two closely related animals of the same family, we have another proof of the close blood-relationship, in the literal sense of the word, of man and the anthropoid ape.

Fig. 203—Lar or white-handed gibbon (Hylobates lar or albimanus), from the Indian mainland (From Brehm.)
The existing anthropoid apes are only a small remnant of a large family of eastern apes (or Catarrhinæ), from which man was evolved about the end of the Tertiary period. They fall into two geographical groups—the Asiatic and the African anthropoids. In each group we can distinguish two genera. The oldest of these four genera is the gibbon Hylobates, Fig. 203); there are from eight to twelve species of it in the East Indies. I made observations of four of them during my voyage in the East Indies (1901), and had a specimen of the ash-grey gibbon (Hylobates leuciscus) living for several months in the garden of my house in Java. I have described the interesting habits of this ape (regarded by the Malays as the wild descendant of men who had lost their way) in my Malayischen Reisebriefen (chap. xi). Psychologically, he showed a good deal of resemblance to the children of my Malay hosts, with whom he played and formed a very close friendship.
The second, larger and stronger, genus of Asiatic anthropoid ape is the orang (Satyrus); he is now found only in the islands of Borneo and Sumatra. Selenka, who has published a very thorough Study of the Development and Cranial Structure of the Anthropoid Apes (1899), distinguishes ten races of the orang, which may, however, also be regarded as “local varieties or species.” They fall into two sub-genera or genera: one group, Dyssatyrus (orang-bentang, Fig. 205), is distinguished for the strength of its limbs, and the formation of very peculiar and salient cheek-pads in the elderly male; these are wanting in the other group, the ordinary orang-outang (Eusatyrus).
Several species have lately been distinguished in the two genera of the black African anthropoid apes (chimpanzee and gorilla). In the genus Anthropithecus (or Anthropopithecus, formerly Troglodytes), the bald-headed chimpanzee, A. calvus (Fig. 206), and the gorilla-like A. mafuca differ very strikingly from the ordinary Anthropithecus niger (Fig. 207), not only in the size and proportion of many parts of the body, but also in the peculiar shape of the head, especially the ears and lips, and in the hair and colour. The controversy that still continues as to whether these different forms of chimpanzee and orang are “merely local varieties” or “true species” is an idle one; as in all such disputes of classifiers there is an utter absence of clear ideas as to what a species really is.
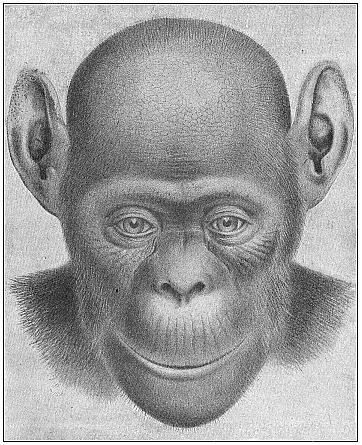
Fig. 206—The bald-headed chimpanzee (Anthropithecus calvus). Female. This fresh species, described by Frank Beddard in 1897 as Troglodytes calvus, differs considerably from the ordinary A. niger Fig. 207) in the structure of the head, the colouring, and the absence of hair in parts.
Of the largest and most famous of all the anthropoid apes, the gorilla, Paschen has lately discovered a giant-form in the interior of the Cameroons, which seems to differ from the ordinary species (Gorilla gina Fig. 208), not only by its unusual size and strength, but also by a special formation of the skull. This giant gorilla (Gorilla gigas, Fig. 209) is six feet eight inches long; the span of its great arms is about nine feet; its powerful chest is twice as broad as that of a strong man.
The whole structure of this huge anthropoid ape is not merely very similar to that of man, but it is substantially the same. “The same 200 bones, arranged in the same way, form our internal skeleton; the same 300 muscles effect our movements; the same hair covers our skin; the same groups of ganglionic cells compose the ingenious mechanism of our brain; the same four-chambered heart is the central pump of our circulation.” The really existing differences in the shape and size of the various parts are explained by differences in their growth, due to adaptation to different habits of life and unequal use of the various organs. This of itself proves morphologically the descent of man from the ape. We will return to the point in Chapter XXIII. But I wanted to point already to this important solution of “the question of questions,” because that agreement in the formation of the embryonic membranes and in fœtal circulation which I have described affords a particularly weighty proof of it. It is the more instructive as even cenogenetic structures may in certain circumstances have a high phylogenetic value. In conjunction with the other facts, it affords a striking confirmation of our biogenetic law.
In turning from the embryology to the phylogeny of man—from the development of the individual to that of the species—we must bear in mind the direct causal connection that exists between these two main branches of the science of human evolution. This important causal nexus finds its simplest expression in “the fundamental law of organic development,” the content and purport of which we have fully considered in the first chapter. According to this biogenetic law, ontogeny is a brief and condensed recapitulation of phylogeny. If this compendious reproduction were complete in all cases, it would be very easy to construct the whole story of evolution on an embryonic basis. When we wanted to know the ancestors of any higher organism, and, therefore, of man—to know from what forms the race as a whole has been evolved we should merely have to follow the series of forms in the development of the individual from the ovum; we could then regard each of the successive forms as the representative of an extinct ancestral form. However, this direct application of ontogenetic facts to phylogenetic ideas is possible, without limitations, only in a very small section of the animal kingdom. There are, it is true, still a number of lower invertebrates (for instance, some of the Zoophyta and Vermalia) in which we are justified in recognising at once each embryonic form as the historical reproduction, or silhouette, as it were, of an extinct ancestor. But in the great majority of the animals, and in the case of man, this is impossible, because the embryonic forms themselves have been modified through the change of the conditions of existence, and have lost their original character to some extent. During the immeasurable course of organic history, the many millions of years during which life was developing on our planet, secondary changes of the embryonic forms have taken place in most animals. The young of animals (not only detached larvæ, but also the embryos enclosed in the womb) may be modified by the influence of the environment, just as well as the mature organisms are by adaptation to the conditions of life; even species are altered during the embryonic development. Moreover, it is an advantage for all higher organisms (and the advantage is greater the more advanced they are) to curtail and simplify the original course of development, and thus to obliterate the traces of their ancestors. The higher the individual organism is in the animal kingdom, the less completely does it reproduce in its embryonic development the series of its ancestors, for reasons that are as yet only partly known to us. The fact is easily proved by comparing the different developments of higher and lower animals in any single stem.
In order to appreciate this important feature, we have distributed the embryological phenomena in two groups, palingenetic and cenogenetic. Under palingenesis we count those facts of embryology that we can directly regard as a faithful synopsis of the corresponding stem-history. By cenogenesis we understand those embryonic processes which we cannot directly correlate with corresponding evolutionary processes, but must regard as modifications or falsifications of them. With this careful discrimination between palingenetic and cenogenetic phenomena, our biogenetic law assumes the following more precise shape:—The rapid and brief development of the individual (ontogeny) is a condensed synopsis of the long and slow history of the stem (phylogeny): this synopsis is the more faithful and complete in proportion as the original features have been preserved by heredity, and modifications have not been introduced by adaptation.
In order to distinguish correctly between palingenetic and cenogenetic phenomena in embryology, and deduce sound conclusions in connection with stem-history, we must especially make a comparative study of the former. In doing this it is best to employ the methods that have long been used by geologists for the purpose of establishing the succession of the sedimentary rocks in the crust of the earth. This solid crust, which encloses the glowing central mass like a thin shell, is composed of different kinds of rocks: there are, firstly, the volcanic rocks which were formed directly by the cooling at the surface of the molten mass of the earth; secondly, there are the sedimentary rocks, that have been made out of the former by the action of water, and have been laid in successive strata at the bottom of the sea. Each of these sedimentary strata was at first a soft layer of mud; but in the course of thousands of years it condensed into a solid, hard mass of stone (sandstone, limestone, marl, etc.), and at the same time permanently preserved the solid and imperishable bodies that had chanced to fall into the soft mud. Among these bodies, which were either fossilised or left characteristic impressions of their forms in the soft slime, we have especially the more solid parts of the animals and plants that lived and died during the deposit of the slimy strata.
Hence each of the sedimentary strata has its characteristic fossils, the remains of the animals and plants that lived during that particular period of the earth’s history. When we make a comparative study of these strata, we can survey the whole series of such periods. All geologists are now agreed that we can demonstrate a definite historical succession in the strata, and that the lowest of them were deposited in very remote, and the uppermost in comparatively recent, times. However, there is no part of the earth where we find the series of strata in its entirety, or even approximately complete. The succession of strata and of corresponding historical periods generally given in geology is an ideal construction, formed by piecing together the various partial discoveries of the succession of strata that have been made at different points of the earth’s surface (cf. Chapter XVIII).
We must act in this way in constructing the phylogeny of man. We must try to piece together a fairly complete picture of the series of our ancestors from the various phylogenetic fragments that we find in the different groups of the animal kingdom. We shall see that we are really in a position to form an approximate picture of the evolution of man and the mammals by a proper comparison of the embryology of very different animals—a picture that we could never have framed from the ontogeny of the mammals alone. As a result of the above-mentioned cenogenetic processes—those of disturbed and curtailed heredity—whole series of lower stages have dropped out in the embryonic development of man and the other mammals especially from the earliest periods, or been falsified by modification. But we find these lower stages in their original purity in the lower vertebrates and their invertebrate ancestors. Especially in the lowest of all the vertebrates, the lancelet or Amphioxus, we have the oldest stem-forms completely preserved in the embryonic development. We also find important evidence in the fishes, which stand between the lower and higher vertebrates, and throw further light on the course of evolution in certain periods. Next to the fishes come the amphibia, from the embryology of which we can also draw instructive conclusions. They represent the transition to the higher vertebrates, in which the middle and older stages of ancestral development have been either distorted or curtailed, but in which we find the more recent stages of the phylogenetic process well preserved in ontogeny. We are thus in a position to form a fairly complete idea of the past development of man’s ancestors within the vertebrate stem by putting together and comparing the embryological developments of the various groups of vertebrates. And when we go below the lowest vertebrates and compare their embryology with that of their invertebrate relatives, we can follow the genealogical tree of our animal ancestors much farther, down to the very lowest groups of animals.
In entering the obscure paths of this phylogenetic labyrinth, clinging to the Ariadne-thread of the biogenetic law and guided by the light of comparative anatomy, we will first, in accordance with the methods we have adopted, discover and arrange those fragments from the manifold embryonic developments of very different animals from which the stem-history of man can be composed. I would call attention particularly to the fact that we can employ this method with the same confidence and right as the geologist. No geologist has ever had ocular proof that the vast rocks that compose our Carboniferous or Jurassic or Cretaceous strata were really deposited in water. Yet no one doubts the fact. Further, no geologist has ever learned by direct observation that these various sedimentary formations were deposited in a certain order; yet all are agreed as to this order. This is because the nature and origin of these rocks cannot be rationally understood unless we assume that they were so deposited. These hypotheses are universally received as safe and indispensable “geological theories,” because they alone give a rational explanation of the strata.
Our evolutionary hypotheses can claim the same value, for the same reasons. In formulating them we are acting on the same inductive and deductive methods, and with almost equal confidence, as the geologist. We hold them to be correct, and claim the status of “biological theories” for them, because we cannot understand the nature and origin of man and the other organisms without them, and because they alone satisfy our demand for a knowledge of causes. And just as the geological hypotheses that were ridiculed as dreams at the beginning of the nineteenth century are now universally admitted, so our phylogenetic hypotheses, which are still regarded as fantastic in certain quarters, will sooner or later be generally received. It is true that, as will soon appear, our task is not so simple as that of the geologist. It is just as much more difficult and complex as man’s organisation is more elaborate than the structure of the rocks.
When we approach this task, we find an auxiliary of the utmost importance in the comparative anatomy and embryology of two lower animal-forms. One of these animals is the lancelet (Amphioxus), the other the sea-squirt (Ascidia). Both of these animals are very instructive. Both are at the border between the two chief divisions of the animal kingdom—the vertebrates and invertebrates. The vertebrates comprise the already mentioned classes, from the Amphioxus to man (acrania, lampreys, fishes, dipneusts, amphibia, reptiles, birds, and mammals). Following the example of Lamarck, it is usual to put all the other animals together under the head of invertebrates. But, as I have often mentioned already, the group is composed of a number of very different stems. Of these we have no interest just now in the echinoderms, molluscs, and articulates, as they are independent branches of the animal-tree, and have nothing to do with the vertebrates. On the other hand, we are greatly concerned with a very interesting group that has only recently been carefully studied, and that has a most important relation to the ancestral tree of the vertebrates. This is the stem of the Tunicates. One member of this group, the sea-squirt, very closely approaches the lowest vertebrate, the Amphioxus, in its essential internal structure and embryonic development. Until 1866 no one had any idea of the close connection of these apparently very different animals; it was a very fortunate accident that the embryology of these related forms was discovered just at the time when the question of the descent of the vertebrates from the invertebrates came to the front. In order to understand it properly, we must first consider these remarkable animals in their fully-developed forms and compare their anatomy.
We begin with the lancelet—after man the most important and interesting of all animals. Man is at the highest summit, the lancelet at the lowest root, of the vertebrate stem.
It lives on the flat, sandy parts of the Mediterranean coast, partly buried in the sand, and is apparently found in a number of seas.[28] It has been found in the North Sea (on the British and Scandinavian coasts and in Heligoland), and at various places on the Mediterranean (for instance, at Nice, Naples, and Messina). It is also found on the coast of Brazil and in the most distant parts of the Pacific Ocean (the coast of Peru, Borneo, China, Australia, etc.). Recently eight to ten species of the amphioxus have been determined, distributed in two or three genera.
[28] See the ample monograph by Arthur Willey, Amphioxus and the Ancestry of the Vertebrates; Boston, 1894.
Johannes Müller classed the lancelet with the fishes, although he pointed out that the differences between this simple vertebrate and the lowest fishes are much greater than between the fishes and the amphibia. But this was far from expressing the real significance of the animal. We may confidently lay down the following principle: The Amphioxus differs more from the fishes than the fishes do from man and the other vertebrates. As a matter of fact, it is so different from all the other vertebrates in its whole organisation that the laws of logical classification compel us to distinguish two divisions of this stem: 1, the Acrania (Amphioxus and its extinct relatives); and 2, the Craniota (man and the other vertebrates). The first and lower division comprises the vertebrates that have no vertebræ or skull (cranium). Of these the only living representatives are the Amphioxus and Paramphioxus, though there must have been a number of different species at an early period of the earth’s history.
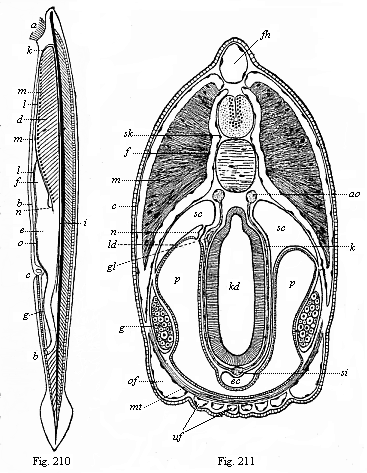
Fig. 210—The lancelet (Amphioxus
lanceolatus), left view. The long axis is vertical; the mouth-end is above,
the tail-end below; a mouth, surrounded by threads of beard; b
anus, c gill-opening (porus branchialis), d gill-crate,
e stomach, f liver, g small intestine, h branchial
cavity, i chorda (axial rod), underneath it the aorta; k aortic
arches, l trunk of the branchial artery, m swellings on its
branches, n vena cava, o visceral vein.
Fig. 211—Transverse section of the head of the
Amphioxus. (From Boveri.) Above the branchial gut (kd) is the
chorda, above this the neural tube (in which we can distinguish the inner grey
and the outer white matter); above again is the dorsal fin (fh). To the
right and left above (in the episoma) are the thick muscular plates (m);
below (in the hyposoma) the gonads (g). ao aorta (here double),
c corium, ec endostyl, f fascie, gl glomerulus of
the kidneys, k branchial vessel, ld partition between the cœloma
(sc) and atrium (p), mt transverse ventral muscle,
n renal canals, of upper and uf lower canals in the mantle-folds,
p peribranchial cavity, (atrium), sc cœloma (subchordal
body-cavity), si principal (or subintestinal) vein, sk perichorda
(skeletal layer).
Opposed to the Acrania is the second division of the vertebrates, which comprises all the other members of the stem, from the fishes up to man. All these vertebrates have a head quite distinct from the trunk, with a skull (cranium) and brain; all have a centralised heart, fully-formed kidneys, etc. Hence they are called the Craniota. These Craniotes are, however, without a skull in their earlier period. As we already know from embryology, even man, like every other mammal, passes in the earlier course of his development through the important stage which we call the chordula; at this lower stage the animal has neither vertebræ nor skull nor limbs (Figs. 83–86). And even after the formation of the primitive vertebræ has begun, the segmented fœtus of the amniotes still has for a long time the simple form of a lyre-shaped disk or a sandal, without limbs or extremities. When we compare this embryonic condition, the sandal-shaped fœtus, with the developed lancelet, we may say that the amphioxus is, in a certain sense, a permanent sandal-embryo, or a permanent embryonic form of the Acrania; it never rises above a low grade of development which we have long since passed.
The fully-developed lancelet (Fig. 210) is about two inches long, is colourless or of a light red tint, and has the shape of a narrow lancet-formed leaf. The body is pointed at both ends, but much compressed at the sides. There is no trace of limbs. The outer skin is very thin and delicate, naked, transparent, and composed of two different layers, a simple external stratum of cells, the epidermis, and a thin underlying cutis-layer. Along the middle line of the back runs a narrow fin-fringe which expands behind into an oval tail-fin, and is continued below in a short anus-fin. The fin-fringe is supported by a number of square elastic fin-plates.
In the middle of the body we find a thin string of cartilage, which goes the whole length of the body from front to back, and is pointed at both ends (Fig. 210 i). This straight, cylindrical rod (somewhat compressed for a time) is the axial rod or the chorda dorsalis; in the lancelet this is the only trace of a vertebral column. The chorda develops no further, but retains its original simplicity throughout life. It is enclosed by a firm membrane, the chorda-sheath or perichorda. The real features of this and of its dependent formations are best seen in the transverse section of the Amphioxus (Fig. 211). The perichorda forms a cylindrical tube immediately over the chorda, and the central nervous system, the medullary tube, is enclosed in it. This important psychic organ also remains in its simplest shape throughout life, as a cylindrical tube, terminating with almost equal plainness at either end, and enclosing a narrow canal in its thick wall. However, the fore end is a little rounder, and contains a small, almost imperceptible bulbous swelling of the canal. This must be regarded as the beginning of a rudimentary brain. At the foremost end of it there is a small black pigment-spot, a rudimentary eye; and a narrow canal leads to a superficial sense-organ. In the vicinity of this optic spot we find at the left side a small ciliated depression, the single olfactory organ. There is no organ of hearing. This defective development of the higher sense-organs is probably, in the main, not an original feature, but a result of degeneration.
Underneath the axial rod or chorda runs a very simple alimentary canal, a tube that opens on the ventral side of the animal by a mouth in front and anus behind. The oval mouth is surrounded by a ring of cartilage, on which there are twenty to thirty cartilaginous threads (organs of touch, Fig. 210 a). The alimentary canal divides into sections of about equal length by a constriction in the middle. The fore section, or head-gut, serves for respiration; the hind section, or trunk-gut, for digestion. The limit of the two alimentary regions is also the limit of the two parts of the body, the head and the trunk. The head-gut or branchial gut forms a broad gill-crate, the grilled wall of which is pierced by numbers of gill-clefts (Fig. 210 d). The fine bars of the gill-crate between the clefts are strengthened with firm parallel rods, and these are connected in pairs by cross-rods. The water that enters the mouth of the Amphioxus passes through these clefts into the large surrounding branchial cavity or atrium, and then pours out behind through a hole in it, the respiratory pore (porus branchialis, Fig. 210 c). Below, on the ventral side of the gill-crate, there is in the middle line a ciliated groove with a glandular wall (the hypobranchial groove), which is also found in the Ascidia and the larvæ of the Cyclostoma. It is interesting because the thyroid gland in the larynx of the higher vertebrates (underneath the “Adam’s apple”) has been developed from it.
Behind the respiratory part of the gut we have the digestive section, the trunk or liver (hepatic) gut. The small particles that the Amphioxus takes in with the water—infusoria, diatoms, particles of decomposed plants and animals, etc.—pass from the gill-crate into the digestive part of the canal, and are used up as food. From a somewhat enlarged portion, that corresponds to the stomach (Fig. 210 e), a long, pouch-like blind sac proceeds straight forward (f); it lies underneath on the left side of the gill-crate, and ends blindly about the middle of it. This is the liver of the Amphioxus, the simplest kind of liver that we meet in any vertebrate. In man also the liver develops, as we shall see, in the shape of a pouch-like blind sac, that forms out of the alimentary canal behind the stomach.
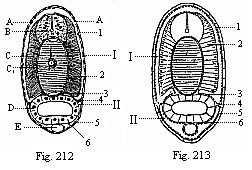
Fig.
212—Transverse section of an Amphioxus-larva, with five
gill-clefts, through the middle of the body.
Fig. 213—Diagram of
the preceding. (From Hatschek.) A epidermis, B
medullary tube, C chorda, C1 inner chorda-sheath,
D visceral epithelium, E sub-intestinal vein. 1 cutis,
2 muscle-plate (myotome), 3 skeletal plate (sclerotome), 4
cœloseptum (partition between dorsal and ventral cœloma), 5 skin-fibre
layer, 6 gut-fibre layer, I myocœl (dorsal body-cavity),
II splanchnocœl (ventral body-cavity).)
The formation of the circulatory system in this animal is not less interesting. All the other vertebrates have a compressed, thick, pouch-shaped heart, which develops from the wall of the gut at the throat, and from which the blood-vessels proceed; in the Amphioxus there is no special centralised heart, driving the blood by its pulsations. This movement is effected, as in the annelids, by the thin blood-vessels themselves, which discharge the function of the heart, contracting and pulsating in their whole length, and thus driving the colourless blood through the entire body. On the under-side of the gill-crate, in the middle line, there is the trunk of a large vessel that corresponds to the heart of the other vertebrates and the trunk of the branchial artery that proceeds from it; this drives the blood into the gills (Fig. 210 l). A number of small vascular arches arise on each side from this branchial artery, and form little heart-shaped swellings or bulbilla (m) at their points of departure; they advance along the branchial arches, between the gill-clefts and the fore-gut, and unite, as branchial veins, above the gill-crate in a large trunk blood-vessel that runs under the chorda dorsalis. This is the principal artery or primitive aorta (Fig. 214 D). The branches which it gives off to all parts of the body unite again in a larger venous vessel at the underside of the gut, called the subintestinal vein (Figs. 210 o, 212 E). This single main vessel of the Amphioxus goes like a closed circular water-conduit along the alimentary canal through the whole body, and pulsates in its whole length above and below. When the upper tube contracts the lower one is filled with blood, and vice versa. In the upper tube the blood flows from front to rear, then back from rear to front in the lower vessel. The whole of the long tube that runs along the ventral side of the alimentary canal and contains venous blood may be called the “principal vein,” and may be compared to the ventral vessel in the worms. On the other hand, the long straight vessel that runs along the dorsal line of the gut above, between it and the chorda, and contains arterial blood, is clearly identical with the aorta or principal artery of the other vertebrates; and on the other side it may be compared to the dorsal vessel in the worms.
The cœloma or body-cavity has some very important and distinctive features in the Amphioxus. The embryology of it is most instructive in connection with the stem-history of the body-cavity in man and the other vertebrates. As we have already seen (Chapter X), in these the two cœlom-pouches are divided at an early stage by transverse constrictions into a double row of primitive segments (Fig. 124), and each of these subdivides, by a frontal or lateral constriction, into an upper (dorsal) and lower (ventral) pouch.
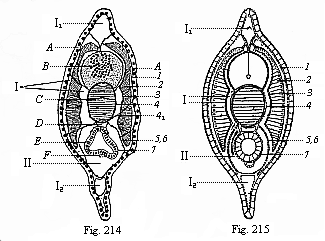
Fig. 214—Transverse section of a young
Amphioxus, immediately after metamorphosis, through the hindermost third
(between the atrium-cavity and the anus).
Fig. 215—Diagram of
preceding. (From Hatschek.) A epidermis, B medullary
tube, C chorda, D aorta, E visceral epithelium, F
subintestinal vein. 1 corium-plate, 2 muscle-plate, 3
fascie-plate, 4 outer chorda-sheath, 5 myoseptum, 6
skin-fibre plate, 7 gut-fibre plate, I myocœl, II
splanchnocœl, I1 dorsal fin, I2
anus-fin.)
These important structures are seen very clearly in the trunk of the amphioxus (the latter third, Figs. 212–215), but it is otherwise in the head, the foremost third (Fig. 216). Here we find a number of complicated structures that cannot be understood until we have studied them on the embryological side in the next chapter (cf. Fig. 81). The branchial gut lies free in a spacious cavity filled with water, which was wrongly thought formerly to be the body-cavity (Fig. 216 A). As a matter of fact, this atrium (commonly called the peribranchial cavity) is a secondary structure formed by the development of a couple of lateral mantle-folds or gill-covers (M1, U). The real body-cavity (Lh) is very narrow and entirely closed, lined with epithelium. The peribranchial cavity (A) is full of water, and its walls are lined with the skin-sense layer; it opens outwards in the rear through the respiratory pore (Fig. 210 c).
On the inner surface of these mantle-folds (M1), in the ventral half of the wide mantle cavity (atrium), we find the sex-organs of the Amphioxus. At each side of the branchial gut there are between twenty and thirty roundish four-cornered sacs, which can clearly be seen from without with the naked eye, as they shine through the thin transparent body-wall. These sacs are the sexual glands they are the same size and shape in both sexes, only differing in contents. In the female they contain a quantity of simple ova (Fig. 219 g); in the male a number of much smaller cells that change into mobile ciliated cells (sperm-cells). Both sacs lie on the inner wall of the atrium, and have no special outlets. When the ova of the female and the sperm of the male are ripe, they fall into the atrium, pass through the gill-clefts into the fore-gut, and are ejected through the mouth.
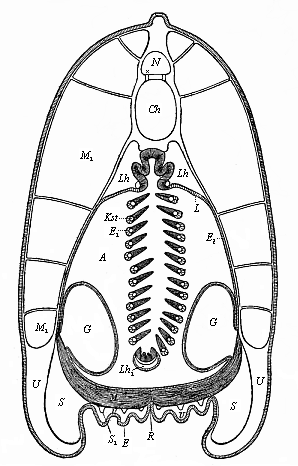
Fig. 216—Transverse section of the lancelet, in the fore half. (From Ralph.) The outer covering is the simple cell-layer of the epidermis (E). Under this is the thin corium, the subcutaneous tissue of which is thickened; it sends connective-tissue partitions between the muscles (M1) and to the chorda-sheath. (N medullary tube, Ch chorda, Lh body-cavity, A atrium, L upper wall of same, E1 inner wall, E2 outer wall, Lh1 ventral remnant of same, Kst gill-reds, M ventral muscles, R seam of the joining of the ventral folds (gill-covers), G sexual glands.
Above the sexual glands, at the dorsal angle of the atrium, we find the kidneys. These important excretory organs could not be found in the Amphioxus for a long time, on account of their remote position and their smallness; they were discovered in 1890 by Theodor Boveri (Fig. 217 x). They are short segmented canals; corresponding to the primitive kidneys of the other vertebrates (Fig. 218 B). Their internal aperture (Fig. 217 B) opens into the body-cavity; their outer aperture into the atrium (C). The prorenal canals lie in the middle of the line of the head, outwards from the uppermost section of the gill-arches, and have important relations to the branchial vessels (H). For this reason, and in their whole arrangement, the primitive kidneys of the Amphioxus show clearly that they are equivalent to the prorenal canals of the Craniotes (Fig. 218 B). The prorenal duct of the latter (Fig. 218 C) corresponds to the branchial cavity or atrium of the former (Fig. 217 C).
Fig. 217—Transverse section through the middle
of the Amphioxus. (From Boveri.) On the left a gill-rod has been
struck, and on the right a gill-cleft; consequently on the left we see the
whole of a prorenal canal (x), on the right only the section of its
fore-leg. A genital chamber (ventral section of the gonocœl), x
pronephridium, B its cœlom-aperture, C atrium, D
body-cavity, E visceral cavity, F subintestinal vein, G
aorta (the left branch connected by a branchial vessel with the subintestinal
vein), H renal vessel.
Fig. 218—Transverse section of a
primitive fish embryo (Selachii-embryo, from Boveri.). To the left
pronephridia (B), the right primitive kidneys (A). The dotted
lines on the right indicate the later opening of the primitive kidney canals
(A) into the prorenal duct (C). D body-cavity, E
visceral cavity, F subintestinal vein, G aorta, H renal
vessel.
If we sum up the results of our anatomic study of the Amphioxus, and compare them with the familiar organisation of man, we shall find an immense distance between the two. As a fact, the highest summit of the vertebrate organisation which man represents is in every respect so far above the lowest stage, at which the lancelet remains, that one would at first scarcely believe it possible to class both animals in the same division of the animal kingdom. Nevertheless, this classification is indisputably just. Man is only a more advanced stage of the vertebral type that we find unmistakably in the Amphioxus in its characteristic features. We need only recall the picture of the ideal Primitive Vertebrate given in a former chapter, and compare it with the lower stages of human embryonic development, to convince ourselves of our close relationship to the lancelet. (Cf. Chapter XI)
It is true that the Amphioxus is far below all other living vertebrates. It is true that it has no separate head, no developed brain or skull, the characteristic feature of the other vertebrates. It is (probably as a result of degeneration) without the auscultory organ and the centralised heart that all the others have; and it has no fully-formed kidneys. Every single organ in it is simpler and less advanced than in any of the others. Yet the characteristic connection and arrangement of all the organs is just the same as in the other vertebrates. All these, moreover, pass, during their embryonic development, through a stage in which their whole organisation is no higher than that of the Amphioxus, but is substantially identical with it.
Fig. 219—Transverse section of the head of the Amphioxus (at the limit of the first and second third of the body). (From Boveri) a aorta (here double), b atrium, c chorda, co umlaut cœloma (body-cavity), e endostyl (hypobranchial groove), g gonads (ovaries), kb gill-arches, kd branchial gut, l liver-tube (on the right, one-sided), m muscles, n renal canals, r spinal cord, sn spinal nerves, sp gill-clefts.
In order to see this quite clearly, it is particularly useful to compare the Amphioxus with the youthful forms of those vertebrates that are classified next to it. This is the class of the Cyclostoma. There are to-day only a few species of this once extensive class, and these may be distributed in two groups. One group comprises the hag-fishes or Myxinoides. The other group are the Petromyzontes, or lampreys, which are a familiar delicacy in their marine form. These Cyclostoma are usually classified with the fishes. But they are far below the true fishes, and form a very interesting connecting-group between them and the lancelet. One can see how closely they approach the latter by comparing a young lamprey with the Amphioxus. The chorda is of the same simple character in both; also the medullary tube, that lies above the chorda, and the alimentary canal below it. However, in the lamprey the spinal cord swells in front into a simple pear-shaped cerebral vesicle, and at each side of it there are a very simple eye and a rudimentary auditory vesicle. The nose is a single pit, as in the Amphioxus. The two sections of the gut are also just the same and very rudimentary in the lamprey. On the other hand, we see a great advance in the structure of the heart, which is found underneath the gills in the shape of a centralised muscular tube, and is divided into an auricle and a ventricle. Later on the lamprey advances still further, and gets a skull, five cerebral vesicles, a series of independent gill-pouches, etc. This makes all the more interesting the striking resemblance of its immature larva to the developed and sexually mature Amphioxus.
While the Amphioxus is thus connected through the Cyclostoma with the fishes, and so with the series of the higher vertebrates, it is, on the other hand, very closely related to a lowly invertebrate marine animal, from which it seems to be entirely remote at first glance. This remarkable animal is the sea-squirt or Ascidia, which was formerly thought to be closely related to the mussel, and so classed in the molluscs. But since the remarkable embryology of these animals was discovered in 1866, there can be no question that they have nothing to do with the molluscs. To the great astonishment of zoologists, they were found, in their whole individual development, to be closely related to the vertebrates. When fully developed the Ascidiæ are shapeless lumps that would not, at first sight, be taken for animals at all. The oval body, frequently studded with knobs or uneven and lumpy, in which we can discover no special external organs, is attached at one end to marine plants, rocks, or the floor of the sea. Many species look like potatoes, others like melon-cacti, others like prunes. Many of the Ascidiæ form transparent crusts or deposits on stones and marine plants. Some of the larger species are eaten like oysters. Fishermen, who know them very well, think they are not animals, but plants. They are sold in the fish markets of many of the Italian coast-towns with other lower marine animals under the name of “sea-fruit” (frutti di mare). There is nothing about them to show that they are animals. When they are taken out of the water with the net the most one can perceive is a slight contraction of the body that causes water to spout out in two places. The bulk of the Ascidiæ are very small, at the most a few inches long. A few species are a foot or more in length. There are many species of them, and they are found in every sea. As in the case of the Acrania, we have no fossilised remains of the class, because they have no hard and fossilisable parts. However, they must be of great antiquity, and must go back to the primordial epoch.
The name of “Tunicates” is given to the whole class to which the Ascidiæ belong, because the body is enclosed in a thick and stiff covering like a mantle (tunica). This mantle—sometimes soft like jelly, sometimes as tough as leather, and sometimes as stiff as cartilage—has a number of peculiarities. The most remarkable of them is that it consists of a woody matter, cellulose—the same vegetal substance that forms the stiff envelopes of the plant-cells, the substance of the wood. The tunicates are the only class of animals that have a real cellulose or woody coat. Sometimes the cellulose mantle is brightly coloured, at other times colourless. Not infrequently it is set with needles or hairs, like a cactus. Often we find a mass of foreign bodies—stone, sand, fragments of mussel-shells, etc.—worked into the mantle. This has earned for the Ascidia the name of “the microcosm.”
Fig. 220—Organisation of an Ascidia (left view); the dorsal side is turned to the right and the ventral side to the left, the mouth (o) above; the ascidia is attached at the tail end. The branchial gut (br), which is pierced by a number of clefts, continues below in the visceral gut. The rectum opens through the anus (a) into the atrium (cl), from which the excrements are ejected with the respiratory water through the mantle-hole or cloaca (a); m mantle. (From Gegenbaur.
The hind end, which corresponds to the tail of the Amphioxus, is usually attached, often by means of regular roots. The dorsal and ventral sides differ a good deal internally, but frequently cannot be distinguished externally. If we open the thick tunic or mantle in order to examine the internal organisation, we first find a spacious cavity filled with water—the mantle-cavity or respiratory cavity (Fig. 220 cl). It is also called the branchial cavity and the cloaca, because it receives the excrements and sexual products as well as the respiratory water. The greater part of the respiratory cavity is occupied by the large grated branchial sac (br). This is so like the gill-crate of the Amphioxus in its whole arrangement that the resemblance was pointed out by the English naturalist Goodsir, years ago, before anything was known of the relationship of the two animals. As a fact, even in the Ascidia the mouth (o) opens first into this wide branchial sac. The respiratory water passes through the lattice-work of the branchial sac into the branchial cavity, and is ejected from this by the respiratory pore (a′). Along the ventral side of the branchial sac runs a ciliated groove—the hypobranchial groove which we have previously found at the same spot in the Amphioxus. The food of the Ascidia also consists of tiny organisms, infusoria, diatoms, parts of decomposed marine plants and animals; etc. These pass with the water into the gill-crate and the digestive part of the gut at the end of it, at first into an enlargement of it that represents the stomach. The adjoining small intestine usually forms a loop, bends forward, and opens by an anus (Fig. 220 a), not directly outwards, but first into the mantle cavity; from this the excrements are ejected by a common outlet (a′) together with the used-up water and the sexual products. The outlet is sometimes called the branchial pore, and sometimes the cloaca or ejection-aperture. In many of the Ascidiæ a glandular mass opens into the gut, and this represents the liver. In some there is another gland besides the liver, and this is taken to represent the kidneys. The body-cavity proper, or cœloma, which is filled with blood and encloses the hepatic gut, is very narrow in the Ascidia, as in the Amphioxus, and is here also usually confounded with the wide atrium, or peribranchial cavity, full of water.
Fig. 221—Organisation of an Ascidia (as in Fig. 220, seen from the left). sb branchial sac, v stomach, i small intestine, c heart, t testicle, vd sperm-duct, o ovary, o′ ripe ova in the branchial cavity. The two small arrows indicate the entrance and exit of the water through the openings of the mantle. (From Milne-Edwards.)
There is no trace in the fully-developed Ascidia of a chorda dorsalis, or internal axial skeleton. It is the more interesting that the young animal that emerges from the ovum has a chorda, and that there is a rudimentary medullary tube above it. The latter is wholly atrophied in the developed Ascidia, and looks like a small nerve-ganglion in front above the gill-crate. It corresponds to the upper “gullet-ganglion” or “primitive brain” in other vermalia. Special sense-organs are either wanting altogether or are only found in a very rudimentary form, as simple optic spots and touch-corpuscles or tentacles that surround the mouth. The muscular system is very slightly and irregularly developed. Immediately under the thin corium, and closely connected with it, we find a thin muscle tube, as in the worms. On the other hand, the Ascidia has a centralised heart, and in this respect it seems to be more advanced than the Amphioxus. On the ventral side of the gut, some distance behind the gill-crate, there is a spindle-shaped heart. It retains permanently the simple tubular form that we find temporarily as the first structure of the heart in the vertebrates. This simple heart of the Ascidia has, however, a remarkable peculiarity. It contracts in alternate directions. In all other animals the beat of the heart is always in the same direction (generally from rear to front); it changes in the Ascidia to the reverse direction. The heart contracts first from the rear to the front, stands still for a minute, and then begins to beat the opposite way, now driving the blood from front to rear; the two large vessels that start from either end of the heart act alternately as arteries and veins. This feature is found in the Tunicates alone.
Of the other chief organs we have still to mention the sexual glands, which lie right behind in the body-cavity. All the Ascidiæ are hermaphrodites. Each individual has a male and a female gland, and so is able to fertilise itself. The ripe ova (Fig. 221 o′) fall directly from the ovary (o) into the mantle-cavity. The male sperm is conducted into this cavity from the testicle (t) by a special duct (vd). Fertilisation is accomplished here, and in many of the Ascidiæ developed embryos are found. These are then ejected with the breathing-water through the cloaca (q), and so “born alive.”
If we now glance at the entire structure of the simple Ascidia (especially Phallusia, Cynthia, etc.) and compare it with that of the Amphioxus, we shall find that the two have few points of contact. It is true that the fully-developed Ascidia resembles the Amphioxus in several important features of its internal structure, and especially in the peculiar character of the gill-crate and gut. But in most other features of organisation it is so far removed from it, and is so unlike it in external appearance, that the really close relationship of the two was not discovered until their embryology was studied. We will now compare the embryonic development of the two animals, and find to our great astonishment that the same embryonic form develops from the ovum of the Amphioxus as from that of the Ascidia—a typical chordula.
The structural features that distinguish the vertebrates from the invertebrates are so prominent that there was the greatest difficulty in the earlier stages of classification in determining the affinity of these two great groups. When scientists began to speak of the affinity of the various animal groups in more than a figurative—in a genealogical—sense, this question came at once to the front, and seemed to constitute one of the chief obstacles to the carrying-out of the evolutionary theory. Even earlier, when they had studied the relations of the chief groups, without any idea of real genealogical connection, they believed they had found here and there among the invertebrates points of contact with the vertebrates: some of the worms, especially, seemed to approach the vertebrates in structure, such as the marine arrow-worm (Sagitta). But on closer study the analogies proved untenable. When Darwin gave an impulse to the construction of a real stem-history of the animal kingdom by his reform of the theory of evolution, the solution of this problem was found to be particularly difficult. When I made the first attempt in my General Morphology (1866) to work out the theory and apply it to classification, I found no problem of phylogeny that gave me so much trouble as the linking of the vertebrates with the invertebrates.
But just at this time the true link was discovered, and at a point where it was least expected. Towards the end of 1866 two works of the Russian zoologist, Kowalevsky, who had lived for some time at Naples, and studied the embryology of the lower animals, were issued in the publications of the St. Petersburg Academy. A fortunate accident had directed the attention of this able observer almost simultaneously to the embryology of the lowest vertebrate, the Amphioxus, and that of an invertebrate, the close affinity of which to the Amphioxus had been least suspected, the Ascidia. To the extreme astonishment of all zoologists who were interested in this important question, there turned out to be the utmost resemblance in structure from the commencement of development between these two very different animals—the lowest vertebrate and the mis-shaped, sessile invertebrate. With this undeniable identity of ontogenesis, which can be demonstrated to an astounding extent, we had, in virtue of the biogenetic law, discovered the long-sought genealogical link, and definitely identified the invertebrate group that represents the nearest blood-relatives of the vertebrates. The discovery was confirmed by other zoologists, and there can no longer be any doubt that of all the classes of invertebrates that of the Tunicates is most closely related to the vertebrates, and of the Tunicates the nearest are the Ascidiæ. We cannot say that the vertebrates are descended from the Ascidiæ—and still less the reverse—but we can say that of all the invertebrates it is the Tunicates, and, within this group, the Ascidiæ, that are the nearest blood-relatives of the ancient stem-form of the vertebrates. We must assume as the common ancestral group of both stems an extinct family of the extensive vermalia-stem, the Prochordonia or Prochordata (“primitive chorda-animals”).
In order to appreciate fully this remarkable fact, and especially to secure the sound basis we seek for the genealogical tree of the vertebrates, it is necessary to study thoroughly the embryology of both these animals, and compare the individual development of the Amphioxus step by step with that of the Ascidia. We begin with the ontogeny of the Amphioxus.
From the concordant observations of Kowalevsky at Naples and Hatschek at Messina, it follows, firstly, that the ovum-segmentation and gastrulation of the Amphioxus are of the simplest character. They take place in the same way as we find them in many of the lower animals of different invertebrate stems, which we have already described as original or primordial; the development of the Ascidia is of the same type. Sexually mature specimens of the Amphioxus, which are found in great quantities at Messina from April or May onwards, begin as a rule to eject their sexual products in the evening; if you catch them about the middle of a warm night and put them in a glass vessel with seawater, they immediately eject through the mouth their accumulated sexual products, in consequence of the disturbance. The males give out masses of sperm, and the females discharge ova in such quantity that many of them stick to the fibrils about their mouths. Both kinds of cells pass first into the mantle-cavity after the opening of the gonads, proceed through the gill-clefts into the branchial gut, and are discharged from this through the mouth.
The ova are simply round cells. They are only 1/250 of an inch in diameter, and thus are only half the size of the mammal ova, and have no distinctive features. The clear protoplasm of the mature ovum is made so turbid by the numbers of dark granules of food-yelk or deutoplasm scattered in it that it is difficult to follow the process of fecundation and the behaviour of the two nuclei during it (p. 51). The active elements of the male sperm, the cone-shaped spermatozoa, are similar to those of most other animals (cf. Fig. 20). Fecundation takes place when these lively ciliated cells of the sperm approach the ovum, and seek to penetrate into the yelk-matter or the cellular substance of the ovum with their head-part—the thicker part of the cell that encloses the nucleus. Only one spermatozoon can bore its way into the yelk at one pole of the ovum-axis; its head or nucleus coalesces with the female nucleus, which remains after the extrusion of the directive bodies from the germinal vesicle. Thus is formed the “stem-nucleus,” or the nucleus of the “stem-cell” (cytula, Fig. 2). This now undergoes total segmentation, dividing into two, four, eight, sixteen, thirty-two cells, and so on. In this way we get the spherical, mulberry-shaped body, which we call the morula.
The segmentation of the Amphioxus is not entirely regular, as was supposed after the first observations of Kowalevsky (1866). It is not completely equal, but a little unequal. As Hatschek afterwards found (1879), the segmentation-cells only remain equal up to the morula-stage, the spherical body of which consists of thirty-two cells. Then, as always happens in unequal segmentation, the more sluggish vegetal cells are outstripped in the cleavage. At the lower or vegetal pole of the ovum a crown of eight large entodermic cells remains for a long time unchanged, while the other cells divide, owing to the formation of a series of horizontal circles, into an increasing number of crowns of sixteen cells each. Afterwards the segmentation-cells get more or less irregularly displaced, while the segmentation-cavity enlarges in the centre of the morula; in the end the former all lie on the surface of the latter, so that the fœtus attains the familiar blastula shape and forms a hollow ball, the wall of which consists of a single stratum of cells (Fig. 38 A–C). This layer is the blastoderm, the simple epithelium from the cells of which all the tissues of the body proceed.
These important early embryonic processes take place so quickly in the Amphioxus that four or five hours after fecundation, or about midnight, the spherical blastula is completed. A pit-like depression is then formed at the vegetal pole of it, and in consequence of this the hollow sphere doubles on itself (Fig. 38 D). This pit becomes deeper and deeper (Fig. 38 E, F); at last the invagination (or doubling) is complete, and the inner or folded part of the blastula-wall lies on the inside of the outer wall. We thus get a hollow hemisphere, the thin wall of which is made up of two layers of cells (Fig. 38 E). From hemispherical the body soon becomes almost spherical once more, and then oval, the internal cavity enlarging considerably and its mouth growing narrower (Fig. 213). The form which the Amphioxus-embryo has thus reached is a real “cup-larva” or gastrula, of the original simple type that we have previously described as the “bell-gastrula” or archigastrula (Figs. 29–35).
As in all the other animals that form an archigastrula, the whole body is nothing but a simple gastric sac or stomach; its internal cavity is the primitive gut (progaster or archenteron, Fig. 38 g, 35 d), and its aperture the primitive mouth (prostoma or blastoporus, o). The wall is at once gut-wall and body-wall. It is composed of two simple cell-layers, the familiar primary germinal layers. The inner layer or the invaginated part of the blastoderm, which immediately encloses the gut-cavity is the entoderm, the inner or vegetal germ-layer, from which develop the wall of the alimentary canal and all its appendages, the cœlom-pouches, etc. (Figs. 35, 36 i). The outer stratum of cells, or the non-invaginated part of the blastoderm, is the ectoderm, the outer or animal germ-layer, which provides the outer skin (epidermis) and the nervous system (e). The cells of the entoderm are much larger, darker, and more fatty than those of the ectoderm, which are clearer and less rich in fatty particles. Hence before and during invagination there is an increasing differentiation of the inner from the outer layer. The animal cells of the outer layer soon develop vibratory hairs; the vegetal cells of the inner layer do so much later. A thread-like process grows out of each cell, and effects continuous vibratory movements. By the vibrations of these slender hairs the gastrula of the Amphioxus swims about in the sea, when it has pierced the thin ovolemma, like the gastrula of many other animals (Fig. 36). As in many other lower animals, the cells have only one whip-like hair each, and so are called flagellate (whip) cells (in contrast with the ciliated cells, which have a number of short lashes or cilia).
In the further course of its rapid development the roundish bell-gastrula becomes elongated, and begins to flatten on one side, parallel to the long axis. The flattened side is the subsequent dorsal side; the opposite or ventral side remains curved. The latter grows more quickly than the former, with the result that the primitive mouth is forced to the dorsal side (Fig. 39). In the middle of the dorsal surface a shallow longitudinal groove or furrow is formed (Fig. 79), and the edges of the body rise up on each side of this groove in the shape of two parallel swellings. This groove is, of course, the dorsal furrow, and the swellings are the dorsal or medullary swellings; they form the first structure of the central nervous system, the medullary tube. The medullary swellings now rise higher; the groove between them becomes deeper and deeper. The edges of the parallel swellings curve towards each other, and at last unite, and the medullary tube is formed (Figs. 83 m, 84 m). Hence the formation of a medullary tube out of the outer skin takes place in the naked dorsal surface of the free-swimming larva of the Amphioxus in just the same way as we have found in the embryo of man and the higher animals within the fœtal membranes.
Simultaneously with the construction of the medullary tube we have in the Amphioxus-embryo the formation of the chorda, the cœlom-pouches, and the mesoderm proceeding from their wall. These processes also take place with characteristic simplicity and clearness, so that they are very instructive to compare with the vermalia on the one hand and with the higher vertebrates on the other. While the medullary groove is sinking in the middle line of the flat dorsal side of the oval embryo, and its parallel edges unite to form the ectodermic neural tube, the single chorda is formed directly underneath them, and on each side of this a parallel longitudinal fold, from the dorsal wall of the primitive gut. These longitudinal folds of the entoderm proceed from the primitive mouth, or from its lower and hinder edge. Here we see at an early stage a couple of large entodermic cells, which are distinguished from all the others by their great size, round form, and fine-grained protoplasm; they are the two promesoblasts, or polar cells of the mesoderm (Fig. 83 p). They indicate the original starting-point of the two cœlom-pouches, which grow from this spot between the inner and outer germinal layers, sever themselves from the primitive gut, and provide the cellular material for the middle layer.
Immediately after their formation the two cœlom-pouches of the Amphioxus are divided into several parts by longitudinal and transverse folds. Each of the primary pouches is divided into an upper dorsal and a lower ventral section by a couple of lateral longitudinal folds (Fig. 82). But these are again divided by several parallel transverse folds into a number of successive sacs, the primitive segments or somites (formerly called by the unsuitable name of “primitive vertebræ”). They have a different future above and below. The upper or dorsal segments, the episomites, lose their cavity later on, and form with their cells the muscular plates of the trunk. The lower or ventral segments, the hyposomites, corresponding to the lateral plates of the craniote-embryo, fuse together in the upper part owing to the disappearance of their lateral walls, and thus form the later body-cavity (metacœl); in the lower part they remain separate, and afterwards form the segmental gonads.
In the middle, between the two lateral cœlom-folds of the primitive gut, a single central organ detaches from this at an early stage in the middle line of its dorsal wall. This is the dorsal chorda (Figs. 83, 84 ch). This axial rod, which is the first foundation of the later vertebral column in all the vertebrates, and is the only representative of it in the Amphioxus, originates from the entoderm.
In consequence of these important folding-processes in the primitive gut, the simple entodermic tube divides into four different sections:— I, underneath, at the ventral side, the permanent alimentary canal or permanent gut; II, above, at the dorsal side, the axial rod or chorda; and III, the two cœlom-sacs, which immediately sub-divide into two structures:—IIIA, above, on the dorsal side, the episomites, the double row of primitive or muscular segments; and IIIB, below, on each side of the gut, the hyposomites, the two lateral plates that give rise to the sex-glands, and the cavities of which partly unite to form the body-cavity. At the same time, the neural or medullary tube is formed above the chorda, on the dorsal surface, by the closing of the parallel medullary swellings. All these processes, which outline the typical structure of the vertebrate, take place with astonishing rapidity in the embryo of the Amphioxus; in the afternoon of the first day, or twenty-four hours after fertilisation, the young vertebrate, the typical embryo, is formed; it then has, as a rule, six to eight somites.
The chief occurrence on the second day of development is the construction of the two permanent openings of the gut—the mouth and anus. In the earlier stages the alimentary tube is found to be entirely closed, after the closing of the primitive mouth; it only communicates behind by the neurenteric canal with the medullary tube. The permanent mouth is a secondary formation, at the opposite end. Here, at the end of the second day, we find a pit-like depression in the outer skin, which penetrates inwards into the closed gut. The anus is formed behind in the same way a few hours later (in the vicinity of the additional gastrula-mouth). In man and the higher vertebrates also the mouth and anus are formed, as we have seen, as flat pits in the outer skin; they then penetrate inwards, gradually becoming connected with the blind ends of the closed gut-tube. During the second day the Amphioxus-embryo undergoes few other changes. The number of primitive segments increases, and generally amounts to fourteen, some forty-eight to fifty hours after impregnation.
Almost simultaneously with the formation of the mouth the first gill-cleft breaks through in the fore section of the Amphioxus-embryo (generally forty hours after the commencement of development). It now begins to nourish itself independently, as the food material stored up in the ovum is completely used up. The further development of the free larvæ takes place very slowly, and extends over several months. The body becomes much longer, and is compressed at the sides, the head-end being broadened in a sort of triangle. Two rudimentary sense-organs are developed in it. Inside we find the first blood-vessels, an upper or dorsal vessel, corresponding to the aorta, between the gut and the dorsal cord, and a lower or ventral vessel, corresponding to the subintestinal vein, at the lower border of the gut. Now, the gills or respiratory organs also are formed at the fore-end of the alimentary canal. The whole of the anterior or respiratory section of the gut is converted into a gill-crate, which is pierced trellis-wise by numbers of branchial-holes, as in the ascidia. This is done by the foremost part of the gut-wall joining star-wise with the outer skin, and the formation of clefts at the point of connection, piercing the wall and leading into the gut from without. At first there are very few of these branchial clefts; but there are soon a number of them—first in one, then in two, rows. The foremost gill-cleft is the oldest. In the end we have a sort of lattice work of fine gill-clefts, supported on a number of stiff branchial rods; these are connected in pairs by transverse rods.
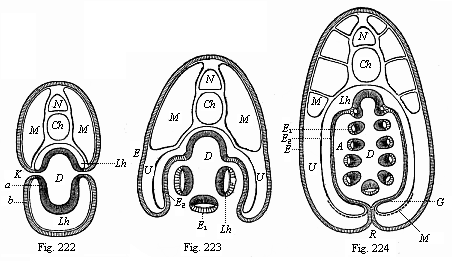
Figs. 222–224—Transverse sections of young Amphioxus-larvæ (diagrammatic, from Ralph.) (Cf. also Fig. 216.) In Fig. 222 there is free communication from without with the gut-cavity (D) through the gill-clefts (K). In Fig. 223 the lateral folds of the body-wall, or the gill-covers, which grow downwards, are formed. In Fig. 224 these lateral folds have united underneath and joined their edges in the middle line of the ventral side (R seam). The respiratory water now passes from the gut-cavity (D) into the mantle-cavity (A). The letters have the same meaning throughout: N medullary tube, Ch chorda, M lateral muscles, Lh body-cavity, G part of the body-cavity in which the sexual organs are subsequently formed. D gut-cavity, clothed with the gut-gland layer (a). A mantle-cavity, K gill-clefts, b=E epidermis, E1 the same as visceral epithelium of the mantle-cavity, E2 as parietal epithelium of the mantle-cavity.
At an early stage of embryonic development the structure of the Amphioxus-larva is substantially the same as the ideal picture we have previously formed of the “Primitive Vertebrate” (Figs. 98–102). But the body afterwards undergoes various modifications, especially in the fore-part. These modifications do not concern us, as they depend on special adaptations, and do not affect the hereditary vertebrate type. When the free-swimming Amphioxus-larva is three months old, it abandons its pelagic habits and changes into the young animal that lives in the sand. In spite of its smallness (one-eighth of an inch), it has substantially the same structure as the adult. As regards the remaining organs of the Amphioxus, we need only mention that the gonads or sexual glands are developed very late, immediately out of the inner cell-layer of the body-cavity. Although we can find afterwards no continuation of the body-cavity (Fig. 216 U) in the lateral walls of the mantle-cavity, in the gill-covers or mantle-folds (Fig. 224 U), there is one present in the beginning (Fig. 224 Lh). The sexual cells are formed below, at the bottom of this continuation (Fig. 224 S). For the rest, the subsequent development into the adult Amphioxus of the larva we have followed is so simple that we need not go further into it here.
We may now turn to the embryology of the Ascidia, an animal that seems to stand so much lower and to be so much more simply organised, remaining for the greater part of its life attached to the bottom of the sea like a shapeless lump. It was a fortunate accident that Kowalevsky first examined just those larger specimens of the Ascidiæ that show most clearly the relationship of the vertebrates to the invertebrates, and the larvæ of which behave exactly like those of the Amphioxus in the first stages of development. This resemblance is so close in the main features that we have only to repeat what we have already said of the ontogenesis of the Amphioxus.
The ovum of the larger Ascidia (Phallusia, Cynthia, etc.) is a simple round cell of 1/250 to 1/125 of an inch in diameter. In the thick fine-grained yelk we find a clear round germinal vesicle of about 1/750 of an inch in diameter, and this encloses a small embryonic spot or nucleolus. Inside the membrane that surrounds the ovum, the stem-cell of the Ascidia, after fecundation, passes through just the same metamorphoses as the stem-cell of the Amphioxus. It undergoes total segmentation; it divides into two, four, eight, sixteen, thirty-two cells, and so on. By continued total cleavage the morula, or mulberry-shaped cluster of cells, is formed. Fluid gathers inside it, and thus we get once more a globular vesicle (the blastula); the wall of this is a single stratum of cells, the blastoderm. A real gastrula (a simple bell-gastrula) is formed from the blastula by invagination, in the same way as in the amphioxus.
Up to this there is no definite ground in the embryology of the Ascidiæ for bringing them into close relationship with the Vertebrates; the same gastrula is formed in the same way in many other animals of different stems. But we now find an embryonic process that is peculiar to the Vertebrates, and that proves irrefragably the affinity of the Ascidiæ to the Vertebrates. From the epidermis of the gastrula a medullary tube is formed on the dorsal side, and, between this and the primitive gut, a chorda; these are the organs that are otherwise only found in Vertebrates. The formation of these very important organs takes place in the Ascidia-gastrula in precisely the same way as in that of the Amphioxus. In the Ascidia (as in the other case) the oval gastrula is first flattened on one side—the subsequent dorsal side. A groove or furrow (the medullary groove) is sunk in the middle line of the flat surface, and two parallel longitudinal swellings arise on either side from the skin layer. These medullary swellings join together over the furrow, and form a tube; in this case, again, the neural or medullary tube is at first open in front, and connected with the primitive gut behind by the neurenteric canal. Further, in the Ascidia-larva also the two permanent apertures of the alimentary canal only appear later, as independent and new formations. The permanent mouth does not develop from the primitive mouth of the gastrula; this primitive mouth closes up, and the later anus is formed near it by invagination from without, on the hinder end of the body, opposite to the aperture of the medullary tube.
During these important processes, that take place in just the same way in the Amphioxus, a tail-like projection grows out of the posterior end of the larva-body, and the larva folds itself up within the round ovolemma in such a way that the dorsal side is curved and the tail is forced on to the ventral side. In this tail is developed—starting from the primitive gut—a cylindrical string of cells, the fore end of which pushes into the body of the larva, between the alimentary canal and the neural canal, and is no other than the chorda dorsalis. This important organ had hitherto been found only in the Vertebrates, not a single trace of it being discoverable in the Invertebrates. At first the chorda only consists of a single row of large entodermic cells. It is afterwards composed of several rows of cells. In the Ascidia-larva, also, the chorda develops from the dorsal middle part of the primitive gut, while the two cœlom-pouches detach themselves from it on both sides. The simple body-cavity is formed by the coalescence of the two.
When the Ascidia-larva has attained this stage of development it begins to move about in the ovolemma. This causes the membrane to burst. The larva emerges from it, and swims about in the sea by means of its oar-like tail. These free-swimming larvæ of the Ascidia have been known for a long time. They were first observed by Darwin during his voyage round the world in 1833. They resemble tadpoles in outward appearance, and use their tails as oars, as the tadpoles do. However, this lively and highly-developed condition does not last long. At first there is a progressive development; the foremost part of the medullary tube enlarges into a brain, and inside this two single sense-organs are developed, a dorsal auditory vesicle and a ventral eye. Then a heart is formed on the ventral side of the animal, or the lower wall of the gut, in the same simple form and at the same spot at which the heart is developed in man and all the other vertebrates. In the lower muscular wall of the gut we find a weal-like thickening, a solid, spindle-shaped string of cells, which becomes hollow in the centre; it begins to contract in different directions, now forward and now backward, as is the case with the adult Ascidia. In this way the sanguineous fluid accumulated in the hollow muscular tube is driven in alternate directions into the blood-vessels, which develop at both ends of the cardiac tube. One principal vessel runs along the dorsal side of the gut, another along its ventral side. The former corresponds to the aorta and the dorsal vessel in the worms. The other corresponds to the subintestinal vein and the ventral vessel of the worms.
Fig. 225—An Appendicaria (Copelata), seen from the left. m mouth, k branchial gut, o gullet, v stomach, a anus, n brain (ganglion above the gullet), g auditory vesicle, f ciliated groove under the gills, h heart, t testicles, e ovary, c chorda, s tail.
With the formation of these organs the progressive development of the Ascidia comes to an end, and degeneration sets in. The free-swimming larva sinks to the floor of the sea, abandons its locomotive habits, and attaches itself to stones, marine plants, mussel-shells, corals, and other objects; this is done with the part of the body that was foremost in movement. The attachment is effected by a number of out-growths, usually three, which can be seen even in the free-swimming larva. The tail is lost, as there is no further use for it. It undergoes a fatty degeneration, and disappears with the chorda dorsalis. The tailless body changes into an unshapely tube, and, by the atrophy of some parts and the modification of others, gradually assumes the appearance we have already described.
Among the living Tunicates there is a very interesting group of small animals that remain throughout life at the stage of development of the tailed, free Ascidia-larva, and swim about briskly in the sea by means of their broad oar-tail. These are the remarkable Copelata (Appendicaria and Vexillaria, Fig. 225). They are the only living Vertebrates that have throughout life a chorda dorsalis and a neural string above it; the latter must be regarded as the prolongation of the cerebral ganglion and the equivalent of the medullary tube. Their branchial gut also opens directly outwards by a pair of branchial clefts. These instructive Copelata, comparable to permanent Ascidia-larvæ, come next to the extinct Prochordonia, those ancient worms which we must regard as the common ancestors of the Tunicates and Vertebrates. The chorda of the Appendicaria is a long, cylindrical string (Fig. 225 c), and serves as an attachment for the muscles that work the flat oar-tail.
Among the various modifications which the Ascidia-larva undergoes after its establishment at the sea-floor, the most interesting (after the loss of the axial rod) is the atrophy of one of its chief organs, the medullary tube. In the Amphioxus the spinal marrow continues to develop, but in the Ascidia the tube soon shrinks into a small and insignificant nervous ganglion that lies above the mouth and the gill-crate, and is in accord with the extremely slight mental power of the animal. This insignificant relic of the medullary tube seems to be quite beyond comparison with the nervous centre of the vertebrate, yet it started from the same structure as the spinal cord of the Amphioxus. The sense-organs that had been developed in the fore part of the neural tube are also lost; no trace of which can be found in the adult Ascidia. On the other hand, the alimentary canal becomes a most extensive organ. It divides presently into two sections—a wide fore or branchial gut that serves for respiration, and a narrower hind or hepatic gut that accomplishes digestion. The branchial or head-gut of the Ascidia is small at first, and opens directly outwards only by a couple of lateral ducts or gill-clefts—a permanent arrangement in the Copelata. The gill-clefts are developed in the same way as in the Amphioxus. As their number greatly increases we get a large gill-crate, pierced like lattice work. In the middle line of its ventral side we find the hypobranchial groove. The mantle or cloaca-cavity (the atrium) that surrounds the gill-crate is also formed in the same way in the Ascidia as in the Amphioxus. The ejection-opening of this peribranchial cavity corresponds to the branchial pore of the Amphioxus. In the adult Ascidia the branchial gut and the heart on its ventral side are almost the only organs that recall the original affinity with the vertebrates.
The further development of the Ascidia in detail has no particular interest for us, and we will not go into it. The chief result that we obtain from its embryology is the complete agreement with that of the Amphioxus in the earliest and most important embryonic stages. They do not begin to diverge until after the medullary tube and alimentary canal, and the axial rod with the muscles between the two, have been formed. The Amphioxus continues to advance, and resembles the embryonic forms of the higher vertebrates; the Ascidia degenerates more and more, and at last, in its adult condition, has the appearance of a very imperfect invertebrate.
If we now look back on all the remarkable features we have encountered in the structure and the embryonic development of the Amphioxus and the Ascidia, and compare them with the features of man’s embryonic development which we have previously studied, it will be clear that I have not exaggerated the importance of these very interesting animals. It is evident that the Amphioxus from the vertebrate side and the Ascidia from the invertebrate form the bridge by which we can span the deep gulf that separates the two great divisions of the animal kingdom. The radical agreement of the lancelet and the sea-squirt in the first and most important stages of development shows something more than their close anatomic affinity and their proximity in classification; it shows also their real blood-relationship and their common origin from one and the same stem-form. In this way, it throws considerable light on the oldest roots of man’s genealogical tree.
Our comparative investigation of the anatomy and ontogeny of the Amphioxus and Ascidia has given us invaluable assistance. We have, in the first place, bridged the wide gulf that has existed up to the present between the Vertebrates and Invertebrates; and, in the second place, we have discovered in the embryology of the Amphioxus a number of ancient evolutionary stages that have long since disappeared from human embryology, and have been lost, in virtue of the law of curtailed heredity. The chief of these stages are the spherical blastula (in its simplest primary form), and the succeeding archigastrula, the pure, original form of the gastrula which the Amphioxus has preserved to this day, and which we find in the same form in a number of Invertebrates of various classes. Not less important are the later embryonic forms of the cœlomula, the chordula, etc.
Thus the embryology of the Amphioxus and the Ascidia has so much increased our knowledge of man’s stem-history that, although our empirical information is still very incomplete, there is now no defect of any great consequence in it. We may now, therefore, approach our proper task, and reconstruct the phylogeny of man in its chief lines with the aid of this evidence of comparative anatomy and ontogeny. In this the reader will soon see the immense importance of the direct application of the biogenetic law. But before we enter upon the work it will be useful to make a few general observations that are necessary to understand the processes aright.
We must say a few words with regard to the period in which the human race was evolved from the animal kingdom. The first thought that occurs to one in this connection is the vast difference between the duration of man’s ontogeny and phylogeny. The individual man needs only nine months for his complete development, from the fecundation of the ovum to the moment when he leaves the maternal womb. The human embryo runs its whole course in the brief space of forty weeks (as a rule, 280 days). In many other mammals the time of the embryonic development is much the same as in man—for instance, in the cow. In the horse and ass it takes a little longer, forty-three to forty-five weeks; in the camel, thirteen months. In the largest mammals, the embryo needs a much longer period for its development in the womb—a year and a half in the rhinoceros, and ninety weeks in the elephant. In these cases pregnancy lasts twice as long as in the case of man, or one and three-quarter years. In the smaller mammals the embryonic period is much shorter. The smallest mammals, the dwarf-mice, develop in three weeks; hares in four weeks, rats and marmots in five weeks, the dog in nine, the pig in seventeen, the sheep in twenty-one and the goat in thirty-six. Birds develop still more quickly. The chick only needs, in normal circumstances, three weeks for its full development. The duck needs twenty-five days, the turkey twenty-seven, the peacock thirty-one, the swan forty-two, and the cassowary sixty-five. The smallest bird, the humming-bird, leaves the egg after twelve days. Hence the duration of individual development within the fœtal membranes is, in the mammals and birds, clearly related to the absolute size of the body of the animal in question. But this is not the only determining feature. There are a number of other circumstances that have an influence on the period of embryonic development. In the Amphioxus the earliest and most important embryonic processes take place so rapidly that the blastula is formed in four hours, the gastrula in six, and the typical vertebrate form in twenty-four.
In every case the duration of ontogeny shrinks into insignificance when we compare it with the enormous period that has been necessary for phylogeny, or the gradual development of the ancestral series. This period is not measured by years or centuries, but by thousands and millions of years. Many millions of years had to pass before the most advanced vertebrate, man, was evolved, step by step, from his ancient unicellular ancestors. The opponents of evolution, who declare that this gradual development of the human form from lower animal forms, and ultimately from a unicellular organism, is an incredible miracle, forget that the same miracle takes place within the space of mine months in the embryonic development of every human being. Each of us has, in the forty weeks—properly speaking, in the first four weeks—of his development in the womb, passed through the same series of transformations that our animal ancestors underwent in the course of millions of years.
It is impossible to determine even approximately, in hundreds or even thousands of years, the real and absolute duration of the phylogenetic period. But for some time now we have, through the research of geologists, been in a position to assign the relative length of the various sections of the organic history of the earth. The immediate data for determining this relative length of the geological periods are found in the thickness of the sedimentary strata—the strata that have been formed at the bottom of the sea or in fresh water from the mud or slime deposited there. These successive layers of limestone, sandstone, slate, marl, etc., which make up the greater part of the rocks, and are often several thousand feet thick, give us a standard for computing the relative length of the various periods.
To make the point quite clear, I must say a word about the evolution of the earth in general, and point out briefly the chief features of the story. In the first place, we encounter the principle that on our planet organic life began to exist at a definite period. That statement is no longer disputed by any competent geologist or biologist. The organic history of the earth could not commence until it was possible for water to settle on our planet in fluid condition. Every organism, without exception, needs fluid water as a condition of existence, and contains a considerable quantity of it. Our own body, when fully formed, contains sixty to seventy per cent of water in its tissues, and only thirty to forty per cent of solid matter. There is even more water in the body of the child, and still more in the embryo. In the earlier stages of development the human fœtus contains more than ninety per cent of water, and not ten per cent of solids. In the lower marine animals, especially certain medusæ, the body consists to the extent of more than ninety-nine per cent of sea-water, and has not one per cent of solid matter. No organism can exist or discharge its functions without water. No water, no life!
But fluid water, on which the existence of life primarily depends, could not exist on our planet until the temperature of the surface of the incandescent sphere had sunk to a certain point. Up to that time it remained in the form of steam. But as soon as the first fluid water could be condensed from the envelope of steam, it began its geological action, and has continued down to the present day to modify the solid crust of the earth. The final outcome of this incessant action of the water—wearing down and dissolving the rocks in the form of rain, hail, snow, and ice, as running stream or boiling surge—is the formation of mud. As Huxley says in his admirable Lectures on the Causes of Phenomena in Organic Nature, the chief document as to the past history of our earth is mud; the question of the history of past ages resolves itself into a question about the formation of mud.
As I have said, it is possible to form an approximate idea of the relative age of the various strata by comparing them at different parts of the earth’s surface. Geologists have long been agreed that there is a definite historical succession of the different strata. The various superimposed layers correspond to successive periods in the organic history of the earth, in which they were deposited in the form of mud at the bottom of the sea. The mud was gradually converted into stone. This was lifted out of the water owing to variations in the earth’s surface, and formed the mountains. As a rule, four or five great divisions are distinguished in the organic history of the earth, corresponding to the larger and smaller groups of the sedimentary strata. The larger periods are then sub-divided into a series of smaller ones, which usually number from twelve to fifteen. The comparative thickness of the groups of strata enables us to make an approximate calculation of the relative length of these various periods of time. We cannot say, it is true, “In a century a stratum of a certain thickness (about two feet) is formed on the average; therefore, a layer 1000 feet thick must be 500,000 years old.” Different strata of the same thickness may need very different periods for their formation. But from the thickness or size of the stratum we can draw some conclusion as to the relative length of the period.
The first and oldest of the four or five chief divisions of the organic history of the earth is called the primordial, archaic, or archeozoic period. If we compute the total average thickness of the sedimentary strata at about 130,000 feet, this first period comprises 70,000 feet, or the greater part of the whole. For this and other reasons we may at once conclude that the corresponding primordial or archeolithic period must have been in itself much longer than the whole of the remaining periods together, from its close to the present day. It was probably much longer than the figures I have quoted (7:6) indicate—possibly 9:6. Of late years the thickness of the archaic rocks has been put at 90,000 feet.
SYNOPSIS OF THE PALEONTOLOGICAL FORMATIONS,
OR THE FOSSILIFEROUS STRATA OF THE CRUST
| Groups | Systems | Formations | Synonyms of
Formations |
|
V. Anthropolithic groups, or anthropozoic (quaternary) groups of strata. | XIV. Recent (alluvium). |
38. Present 37. Recent | Upper
alluvial Lower alluvial |
| XIII. Pleistocene (diluvium) |
36. Post-glacial 35. Glacial | Upper diluvial Lower diluvial | |
|
IV. Cenolithic groups, or cenozoic (tertiary) groups of strata. | XII. Pliocene (neo-tertiary) | 34. Arverne 33. Subapennine | Upper
pliocene Lower pliocene |
| XI. Miocene (middle tertiary) |
32. Falun 31. Limbourg | Upper miocene Lower miocene | |
| Xb. Oligocene (old tertiary) |
30. Aquitaine 29. Ligurium | Upper oligocene Lower oligocene | |
| Xa. Eocene (primitive tertiary) |
28. Gypsum 27. Coarse chalk 26. London clay |
Upper eocene Middle eocene Lower eocene |
|
|
III. Mesolithic groups, or mesozoic (secondary) groups of strata. | IX. Chalk (cretaceous) | 25. White chalk 24. Green sand 23. Neoconian 22. Wealden | Upper cretaceous Middle cretaceous Lower cretaceous Weald formation |
| VIII. Jurassic | 21.
Portland 20. Oxford 19. Bath 18. Lias |
Upper oolithic Middle oolithic Lower oolithic Liassic |
|
| VII. Triassic | 17.
Keuper 16. Muschelkalk 15. Bunter | Upper
triassic Middle triassic Lower triassic | |
|
II. Paleolithic groups, or paleozoic (primary) groups of strata. | VIb. Permian | 14.
Zechstein 13. Neurot sand | Upper permian Lower permian |
| VIa. Carboniferous coal-measures) | 12. Carboniferous sandstone 11. Carboniferous limestone | Upper
carboniferous Lower carboniferous | |
| V. Devonian | 10. Pilton 9. Ilfracombe 8. Linton |
Upper devonian Middle devonian Lower devonian | |
| IV. Silurian |
7. Ludlow 6. Wenlock 5. Llandeilo | Upper silurian Middle silurian Lower silurian | |
|
I. Archeolithic groups, or archeozoic (primordial) groups of strata. | III. Cambrian | 4. Potsdam 3. Longmynd |
Upper cambrian Lower cambrian |
| II. Huronian I. Laurentian | 2. Labrador 1. Ottawa | Upper laurentian Lower laurentian |
The primordial period falls into three subordinate sections—the Laurentian, Huronian, and Cambrian, corresponding to the three chief groups of rocks that comprise the archaic formation. The immense period during which these rocks were forming in the primitive ocean probably comprises more than 50,000,000 years. At the commencement of it the oldest and simplest organisms were formed by spontaneous generation—the Monera, with which the history of life on our planet opened. From these were first developed unicellular organisms of the simplest character, the Protophyta and Protozoa (paulotomea, amœbæ, rhizopods, infusoria, and other Protists). During this period the whole of the invertebrate ancestors of the human race were evolved from the unicellular organisms. We can deduce this from the fact that we already find remains of fossilised fishes (Selachii and Ganoids) towards the close of the following Silurian period. These are much more advanced and much younger than the lowest vertebrate, the Amphioxus, and the numerous skull-less vertebrates, related to the Amphioxus, that must have lived at that time. The whole of the invertebrate ancestors of the human race must have preceded these.
The primordial age is followed by a much shorter division, the paleozoic or Primary age. It is divided into four long periods, the Silurian, Devonian, Carboniferous, and Permian. The Silurian strata are particularly interesting because they contain the first fossil traces of vertebrates—teeth and scales of Selachii ( Palæodus) in the lower, and Ganoids ( Pteraspis) in the upper Silurian. During the Devonian period the “old red sandstone” was formed; during the Carboniferous period were deposited the vast coal-measures that yield us our chief combustive material; in the Permian (or the Dyas), in fine, the new red sandstone, the Zechstein (magnesian limestone), and the Kupferschiefer (marl-slate) were formed. The collective depth of these strata is put at 40,000 to 45,000 feet. In any case, the paleozoic age, taken as a whole, was much shorter than the preceding and much longer than the subsequent periods. The strata that were deposited during this primary epoch contain a large number of fossils; besides the invertebrate species there are a good many vertebrates, and the fishes preponderate. There were so many fishes, especially primitive fishes (of the shark type) and plated fishes, during the Devonian, and also during the Carboniferous and Permian periods, that we may describe the whole paleozoic period as “the age of fishes.” Among the paleozoic plated fishes or Ganoids the Crossopterygii and the Ctenodipterina (dipneusts) are of great importance.
During this period some of the fishes began to adapt themselves to living on land, and so gave rise to the class of the amphibia. We find in the Carboniferous period fossilised remains of five-toed amphibia, the oldest terrestrial, air-breathing vertebrates. These amphibia increase in variety in the Permian epoch. Towards the close of it we find the first Amniotes, the ancestors of the three higher classes of Vertebrates. These are lizard-like animals; the first to be discovered was the Proterosaurus, from the marl at Eisenach. The rise of the earliest Amniotes, among which must have been the common ancestor of the reptiles, birds, and mammals, is put back towards the close of the paleozoic age by the discovery of these reptile remains. The ancestors of our race during this period were at first represented by true fishes, then by dipneusts and amphibia, and finally by the earliest Amniotes, or the Protamniotes.
The third chief section of the organic history of the earth is the Mesozoic or Secondary period. This again is subdivided into three divisions Triassic, Jurassic, and Cretaceous. The thickness of the strata that were deposited in this period, from the beginning of the Triassic to the end of the Cretaceous period, is altogether about 15,000 feet, or not half as much as the paleozoic deposits. During this period there was a very brisk and manifold development in all branches of the animal kingdom. There were especially a number of new and interesting forms evolved in the vertebrate stem. Bony fishes ( Teleostei) make their first appearance. Reptiles are found in extraordinary variety and number; the extinct giant-serpents (dinosauria), the sea-serpents (halisauria), and the flying lizards (pterosauria) are the most remarkable and best known of these. On account of this predominance of the reptile-class, the period is called “the age of reptiles.” But the bird-class was also evolved during this period; they certainly originated from some division of the lizard-like reptiles. This is proved by the embryological identity of the birds and reptiles and their comparative anatomy, and, among other features, from the circumstance that in this period there were birds with teeth in their jaws and with tails like lizards (Archeopteryx, Odontornis).
Finally, the most advanced and (for us) the most important class of the vertebrates, the mammals, made their appearance during the mesozoic period. The earliest fossil remains of them were found in the latest Triassic strata—lower jaws of small ungulates and marsupials. More numerous remains are found a little later in the Jurassic, and some in the Cretaceous. All the mammal remains that we have from this section belong to the lower promammals and marsupials; among these were most certainly the ancestors of the human race. On the other hand, we have not found a single indisputable fossil of any higher mammal (a placental) in the whole of this period. This division of the mammals, which includes man, was not developed until later, towards the close of this or in the following period.
The fourth section of the organic history of the earth, the Tertiary or Cenozoic age, was much shorter than the preceding. The strata that were deposited during this period have a collective thickness of only about 3,000 feet. It is subdivided into four sections—the Eocene, Oligocene, Miocene, and Pliocene. During these periods there was a very varied development of higher plant and animal forms; the fauna and flora of our planet approached nearer and nearer to the character that they bear to-day. In particular, the most advanced class, the mammals, began to preponderate. Hence the Tertiary period may be called “the age of mammals.” The highest section of this class, the placentals, now made their appearance; to this group the human race belongs. The first appearance of man, or, to be more precise, the development of man from some closely-related group of apes, probably falls in either the miocene or the pliocene period, the middle or the last section of the Tertiary period. Others believe that man properly so-called—man endowed with speech—was not evolved from the non-speaking ape-man ( Pithecanthropus) until the following, the anthropozoic, age.
In this fifth and last section of the organic history of the earth we have the full development and dispersion of the various races of men, and so it is called the Anthropozoic as well as the Quaternary period. In the imperfect condition of paleontological and ethnographical science we cannot as yet give a confident answer to the question whether the evolution of the human race from some extinct ape or lemur took place at the beginning of this or towards the middle or the end of the Tertiary period. However, this much is certain: the development of civilisation falls in the anthropozoic age, and this is merely an insignificant fraction of the vast period of the whole history of life. When we remember this, it seems ridiculous to restrict the word “history” to the civilised period. If we divide into a hundred equal parts the whole period of the history of life, from the spontaneous generation of the first Monera to the present day, and if we then represent the relative duration of the five chief sections or ages, as calculated from the average thickness of the strata they contain, as percentages of this, we get something like the following relation:—
| I. II. III. IV. V. | Archeolithic or archeozoic (primordial) age Paleolithic or paleozoic (primary) age Mesolithic or mesozoic (secondary) age Cenolithic or cenozoic (tertiary) age Anthropolithic or anthropozoic (quaternary) age | 53.6 32.1 11.5 2.3 0.5 ——— 100.0 |
In any case, the “historical period” is an insignificant quantity compared with the vast length of the preceding ages, in which there was no question of human existence on our planet. Even the important Cenozoic or Tertiary period, in which the first placentals or higher mammals appear, probably amounts to little over two per cent of the whole organic age.
Before we approach our proper task, and, with the aid of our ontogenetic acquirements and the biogenetic law, follow step by step the paleontological development of our animal ancestors, let us glance for a moment at another, and apparently quite remote, branch of science, a general consideration of which will help us in the solving of a difficult problem. I mean the science of comparative philology. Since Darwin gave new life to biology by his theory of selection, and raised the question of evolution on all sides, it has often been pointed out that there is a remarkable analogy between the development of languages and the evolution of species. The comparison is perfectly just and very instructive. We could hardly find a better analogy when we are dealing with some of the difficult and obscure features of the evolution of species. In both cases we find the action of the same natural laws.
All philologists of any competence in their science now agree that all human languages have been gradually evolved from very rudimentary beginnings. The idea that speech is a gift of the gods—an idea held by distinguished authorities only fifty years ago—is now generally abandoned, and only supported by theologians and others who admit no natural development whatever. Speech has been developed simultaneously with its organs, the larynx and tongue, and with the functions of the brain. Hence it will be quite natural to find in the evolution and classification of languages the same features as in the evolution and classification of organic species. The various groups of languages that are distinguished in philology as primitive, fundamental, parent, and daughter languages, dialects, etc., correspond entirely in their development to the different categories which we classify in zoology and botany as stems, classes, orders, families, genera, species, and varieties. The relation of these groups, partly co-ordinate and partly subordinate, in the general scheme is just the same in both cases; and the evolution follows the same lines in both.
When, with the assistance of this tree, we follow the formation of the various languages that have been developed from the common root of the ancient Indo-Germanic tongue, we get a very clear idea of their phylogeny. We shall see at the same time how analogous this is to the development of the various groups of vertebrates that have arisen from the common stem-form of the primitive vertebrate. The ancient Indo-Germanic root-language divided first into two principal stems—the Slavo-Germanic and the Aryo-Romanic. The Slavo-Germanic stem then branches into the ancient Germanic and the ancient Slavo-Lettic tongues; the Aryo-Romanic into the ancient Aryan and the ancient Greco-Roman. If we still follow the genealogical tree of these four Indo-Germanic tongues, we find that the ancient Germanic divides into three branches—the Scandinavian, the Gothic, and the German. From the ancient German came the High German and Low German; to the latter belong the Frisian, Saxon, and modern Low-German dialects. The ancient Slavo-Lettic divided first into a Baltic and a Slav language. The Baltic gave rise to the Lett, Lithuanian, and old-Prussian varieties; the Slav to the Russian and South-Slav in the south-east, and to the Polish and Czech in the west.
We find an equally prolific branching of its two chief stems when we turn to the other division of the Indo-Germanic languages. The Greco-Roman divided into the Thracian (Albano-Greek) and the Italo-Celtic. From the latter came the divergent branches of the Italic (Roman and Latin) in the south, and the Celtic in the north: from the latter have been developed all the British (ancient British, ancient Scotch, and Irish) and Gallic varieties. The ancient Aryan gave rise to the numerous Iranian and Indian languages.
This “comparative anatomy” and evolution of languages admirably illustrates the phylogeny of species. It is clear that in structure and development the primitive languages, mother and daughter languages, and varieties, correspond exactly to the classes, orders, genera, and species of the animal world. In both cases the “natural” system is phylogenetic. As we have been convinced from comparative anatomy and ontogeny, and from paleontology, that all past and living vertebrates descend from a common ancestor, so the comparative study of dead and living Indo-Germanic tongues proves beyond question that they are all modifications of one primitive language. This view of their origin is now accepted by all the chief philologists who have worked in this branch and are unprejudiced.
But the point to which I desire particularly to draw the reader’s attention in this comparison of the Indo-Germanic languages with the branches of the vertebrate stem is, that one must never confuse direct descendants with collateral branches, nor extinct forms with living. This confusion is very common, and our opponents often make use of the erroneous ideas it gives rise to for the purpose of attacking evolution generally. When, for instance, we say that man descends from the ape, this from the lemur, and the lemur from the marsupial, many people imagine that we are speaking of the living species of these orders of mammals that they find stuffed in our museums. Our opponents then foist this idea on us, and say, with more astuteness than intelligence, that it is quite impossible; or they ask us, by way of physiological experiment, to turn a kangaroo into a lemur, a lemur into a gorilla, and a gorilla into a man! The demand is childish, and the idea it rests on erroneous. All these living forms have diverged more or less from the ancestral form; none of them could engender the same posterity that the stem-form really produced thousands of years ago.
It is certain that man has descended from some extinct mammal; and we should just as certainly class this in the order of apes if we had it before us. It is equally certain that this primitive ape descended in turn from an unknown lemur, and this from an extinct marsupial. But it is just as clear that all these extinct ancestral forms can only be claimed as belonging to the living order of mammals in virtue of their essential internal structure and their resemblance in the decisive anatomic characteristics of each order. In external appearance, in the characteristics of the genus or species, they would differ more or less, perhaps very considerably, from all living representatives of those orders. It is a universal and natural procedure in phylogenetic development that the stem-forms themselves, with their specific peculiarities, have been extinct for some time. The forms that approach nearest to them among the living species are more or less—perhaps very substantially—different from them. Hence in our phylogenetic inquiry and in the comparative study of the living, divergent descendants, there can only be a question of determining the greater or less remoteness of the latter from the ancestral form. Not a single one of the older stem-forms has continued unchanged down to our time.
We find just the same thing in comparing the various dead and living languages that have developed from a common primitive tongue. If we examine our genealogical tree of the Indo-Germanic languages in this light, we see at once that all the older or parent tongues, of which we regard the living varieties of the stem as divergent daughter or grand-daughter languages, have been extinct for some time. The Aryo-Romanic and the Slavo-Germanic tongues have completely disappeared; so also the Aryan, the Greco-Roman, the Slavo-Lettic, and the ancient Germanic. Even their daughters and grand-daughters have been lost; all the living Indo-Germanic languages are only related in the sense that they are divergent descendants of common stem-forms. Some forms have diverged more, and some less, from the original stem-form.
This easily demonstrable fact illustrates very well the analogous case of the origin of the vertebrate species. Phylogenetic comparative philology here yields a strong support to phylogenetic comparative zoology. But the one can adduce more direct evidence than the other, as the paleontological material of philology—the old monuments of the extinct tongue—have been preserved much better than the paleontological material of zoology, the fossilised bones and imprints of vertebrates.
We may, however, trace man’s genealogical tree not only as far as the lower mammals, but much further—to the amphibia, to the shark-like primitive fishes, and, in fine, to the skull-less vertebrates that closely resembled the Amphioxus. But this must not be understood in the sense that the existing Amphioxus, or the sharks or amphibia of to-day, can give us any idea of the external appearance of these remote stem-forms. Still less must it be thought that the Amphioxus or any actual shark, or any living species of amphibia, is a real ancestral form of the higher vertebrates and man. The statement can only rationally mean that the living forms I have referred to are collateral lines that are much more closely related to the extinct stem-forms, and have retained the resemblance much better, than any other animals we know. They are still so like them in regard to their distinctive internal structure that we should put them in the same class with the extinct forms if we had these before us. But no direct descendants of these earlier forms have remained unchanged. Hence we must entirely abandon the idea of finding direct ancestors of the human race in their characteristic external form among the living species of animals. The essential and distinctive features that still connect living forms more or less closely with the extinct common stem-forms lie in the internal structure, not the external appearance. The latter has been much modified by adaptation. The former has been more or less preserved by heredity.
Comparative anatomy and ontogeny prove beyond question that man is a true vertebrate, and, therefore, man’s special genealogical tree must be connected with that of the other Vertebrates, which spring from a common root with him. But we have also many important grounds in comparative anatomy and ontogeny for assuming a common origin for all the Vertebrates. If the general theory of evolution is correct, all the Vertebrates, including man, come from a single common ancestor, a long-extinct “Primitive Vertebrate.” Hence the genealogical tree of the Vertebrates is at the same time that of the human race.
Our task, therefore, of constructing man’s genealogy becomes the larger aim of discovering the genealogy of the entire vertebrate stem. As we now know from the comparative anatomy and ontogeny of the Amphioxus and the Ascidia, this is in turn connected with the genealogical tree of the Invertebrates (directly with that of the Vermalia), but has no direct connection with the independent stems of the Articulates, Molluscs, and Echinoderms. If we do thus follow our ancestral tree through various stages down to the lowest worms, we come inevitably to the Gastræa, that most instructive form that gives the clearest possible picture of an animal with two germinal layers. The Gastræa itself has originated from the simple multicellular vesicle, the Blastæa, and this in turn must have been evolved from the lowest circle of unicellular animals, to which we give the name of Protozoa. We have already considered the most important primitive type of these, the unicellular Amœba, which is extremely instructive when compared with the human ovum. With this we reach the lowest of the solid data to which we are to apply our biogenetic law, and by which we may deduce the extinct ancestor from the embryonic form. The amœboid nature of the young ovum and the unicellular condition in which (as stem-cell or cytula) every human being begins its existence justify us in affirming that the earliest ancestors of the human race were simple amœboid coils.
But the further question now arises: “Whence came these first amœbæ with which the history of life began at the commencement of the Laurentian epoch?” There is only one answer to this. The earliest unicellular organisms can only have been evolved from the simplest organisms we know, the Monera. These are the simplest living things that we can conceive. Their whole body is nothing but a particle of plasm, a granule of living albuminous matter, discharging of itself all the essential vital functions that form the material basis of life. Thus we come to the last, or, if you prefer, the first, question in connection with evolution—the question of the origin of the Monera. This is the real question of the origin of life, or of spontaneous generation.
We have neither space nor occasion to go further in this Chapter into the question of spontaneous generation. For this I must refer the reader to the fifteenth chapter of the History of Creation, and especially to the second book of the General Morphology, or to the essay on “The Monera and Spontaneous Generation” in my Studies of the Monera and other Protists.[29] I have given there fully my own view of this important question. The famous botanist Nägeli afterwards (1884) developed the same ideas. I will only say a few words here about this obscure question of the origin of life, in so far as our main subject, organic evolution in general, is affected by it. Spontaneous generation, in the definite and restricted sense in which I maintain it, and claim that it is a necessary hypothesis in explaining the origin of life, refers solely to the evolution of the Monera from inorganic carbon-compounds. When living things made their first appearance on our planet, the very complex nitrogenous compound of carbon that we call plasson, which is the earliest material embodiment of vital action, must have been formed in a purely chemical way from inorganic carbon-compounds. The first Monera were formed in the sea by spontaneous generation, as crystals are formed in the mother-water. Our demand for a knowledge of causes compels us to assume this. If we believe that the whole inorganic history of the earth has proceeded on mechanical principles without any intervention of a Creator, and that the history of life also has been determined by the same mechanical laws; if we see that there is no need to admit creative action to explain the origin of the various groups of organisms; it is utterly irrational to assume such creative action in dealing with the first appearance of organic life on the earth.
[29] The English reader will find a luminous and up-to-date chapter on the subject in Haeckel’s recently written and translated Wonders of Life.—Translator.
This much-disputed question of “spontaneous generation” seems so obscure, because people have associated with the term a mass of very different, and often very absurd, ideas, and have attempted to solve the difficulty by the crudest experiments. The real doctrine of the spontaneous generation of life cannot possibly be refuted by experiments. Every experiment that has a negative result only proves that no organism has been formed out of inorganic matter in the conditions—highly artificial conditions—we have established. On the other hand, it would be exceedingly difficult to prove the theory by way of experiment; and even if Monera were still formed daily by spontaneous generation (which is quite possible), it would be very difficult, if not impossible, to find a solid proof of it. Those who will not admit the spontaneous generation of the first living things in our sense must have recourse to a supernatural miracle; and this is, as a matter of fact, the desperate resource to which our “exact” scientists are driven, to the complete abdication of reason.
A famous English physicist, Lord Kelvin (then Sir W. Thomson), attempted to dispense with the hypothesis of spontaneous generation by assuming that the organic inhabitants of the earth were developed from germs that came from the inhabitants of other planets, and that chanced to fall on our planet on fragments of their original home, or meteorites. This hypothesis found many supporters, among others the distinguished German physicist, Helmholtz. However, it was refuted in 1872 by the able physicist, Friedrich Zöllner, of Leipzig, in his work, On the Nature of Comets. He showed clearly how unscientific this hypothesis is; firstly in point of logic, and secondly in point of scientific content. At the same time he pointed out that our hypothesis of spontaneous generation is “a necessary condition for understanding nature according to the law of causality.”
I repeat that we must call in the aid of the hypothesis only as regards the Monera, the structureless “organisms without organs.” Every complex organism must have been evolved from some lower organism. We must not assume the spontaneous generation of even the simplest cell, for this itself consists of at least two parts—the internal, firm nuclear substance, and the external, softer cellular substance or the protoplasm of the cell-body. These two parts must have been formed by differentiation from the indifferent plasson of a moneron, or a cytode. For this reason the natural history of the Monera is of great interest; here alone can we find the means to overcome the chief difficulties of the problem of spontaneous generation. The actual living Monera are specimens of such organless or structureless organisms, as they must have boon formed by spontaneous generation at the commencement of the history of life.
Under the guidance of the biogenetic law, and on the basis of the evidence we have obtained, we now turn to the interesting task of determining the series of man’s animal ancestors. Phylogeny us a whole is an inductive science. From the totality of the biological processes in the life of plants, animals, and man we have gathered a confident inductive idea that the whole organic population of our planet has been moulded on a harmonious law of evolution. All the interesting phenomena that we meet in ontogeny and paleontology, comparative anatomy and dysteleology, the distribution and habits of organisms—all the important general laws that we abstract from the phenomena of these sciences, and combine in harmonious unity—are the broad bases of our great biological induction.
But when we come to the application of this law, and seek to determine with its aid the origin of the various species of organisms, we are compelled to frame hypotheses that have essentially a deductive character, and are inferences from the general law to particular cases. But these special deductions are just as much justified and necessitated by the rigorous laws of logic as the inductive conclusions on which the whole theory of evolution is built. The doctrine of the animal ancestry of the human race is a special deduction of this kind, and follows with logical necessity from the general inductive law of evolution.
I must point out at once, however, that the certainty of these evolutionary hypotheses, which rest on clear special deductions, is not always equally strong. Some of these inferences are now beyond question; in the case of others it depends on the knowledge and the competence of the inquirer what degree of certainty he attributes to them. In any case, we must distinguish between the absolute certainty of the general (inductive) theory of descent and the relative certainty of special (deductive) evolutionary hypotheses. We can never determine the whole ancestral series of an organism with the same confidence with which we hold the general theory of evolution as the sole scientific explanation of organic modifications. The special indication of stem-forms in detail will always be more or less incomplete and hypothetical. This is quite natural. The evidence on which we build is imperfect, and always will be imperfect; just as in comparative philology.
The first of our documents, paleontology, is exceedingly incomplete. We know that all the fossils yet discovered are only an insignificant fraction of the plants and animals that have lived on our planet. For every single species that has been preserved for us in the rocks there are probably hundreds, perhaps thousands, of extinct species that have left no trace behind them. This extreme and very unfortunate incompleteness of the paleontological evidence, which cannot be pointed out too often, is easily explained. It is absolutely inevitable in the circumstances of the fossilisation of organisms. It is also due in part to the incompleteness of our knowledge in this branch. It must be borne in mind that the great majority of the stratified rocks that compose the crust of the earth have not yet been opened. We have only a few specimens of the innumerable fossils that are buried in the vast mountain ranges of Asia and Africa. Only a part of Europe and North America has been investigated carefully. The whole of the fossils known to us certainly do not amount to a hundredth part of the remains that are really buried in the crust of the earth. We may, therefore, look forward to a rich harvest in the future as regards this science. However, our paleontological evidence will (for reasons that I have fully explained in the sixteenth chapter of the History of Creation) always be defective.
The second chief source of evidence, ontogeny, is not less incomplete. It is the most important source of all for special phylogeny; but it has great defects, and often fails us. We must, above all, clearly distinguish between palingenetic and cenogenetic phenomena. We must never forget that the laws of curtailed and disturbed heredity often make the original course of development almost unrecognisable. The recapitulation of phylogeny by ontogeny is only fairly complete in a few cases, and is never wholly complete. As a rule, it is precisely the earliest and most important embryonic stages that suffer most from alteration and condensation. The earlier embryonic forms have had to adapt themselves to new circumstances, and so have been modified. The struggle for existence has had just as profound an influence on the freely moving and still immature young forms as on the adult forms. Hence in the embryology of the higher animals, especially, palingenesis is much restricted by cenogenesis; it is to-day, as a rule, only a faded and much altered picture of the original evolution of the animal’s ancestors. We can only draw conclusions from the embryonic forms to the stem-history with the greatest caution and discrimination. Moreover, the embryonic development itself has only been fully studied in a few species.
Finally, the third and most valuable source of evidence, comparative anatomy, is also, unfortunately, very imperfect; for the simple reason that the whole of the living species of animals are a mere fraction of the vast population that has dwelt on our planet since the beginning of life. We may confidently put the total number of these at more than a million species. The number of animals whose organisation has been studied up to the present in comparative anatomy is proportionately very small. Here, again, future research will yield incalculable treasures. But, for the present, in view of this patent incompleteness of our chief sources of evidence, we must naturally be careful not to lay too much stress in human phylogeny on the particular animals we have studied, or regard all the various stages of development with equal confidence as stem-forms.
In my first efforts to construct the series of man’s ancestors I drew up a list of, at first ten, afterwards twenty to thirty, forms that may be regarded more or less certainly as animal ancestors of the human race, or as stages that in a sense mark off the chief sections in the long story of evolution from the unicellular organism to man. Of these twenty to thirty stages, ten to twelve belong to the older group of the Invertebrates and eighteen to twenty to the younger division of the Vertebrates.
In approaching, now, the difficult task of establishing the evolutionary succession of these thirty ancestors of humanity since the beginning of life, and in venturing to lift the veil that covers the earliest secrets of the earth’s history, we must undoubtedly look for the first living things among the wonderful organisms that we call the Monera; they are the simplest organisms known to us—in fact, the simplest we can conceive. Their whole body consists merely of a simple particle or globule of structureless plasm or plasson. The discoveries of the last four decades have led us to believe with increasing certainty that wherever a natural body exhibits the vital processes of nutrition, reproduction, voluntary movement, and sensation, we have the action of a nitrogenous carbon-compound of the chemical group of the albuminoids; this plasm (or protoplasm) is the material basis of all vital functions. Whether we regarded the function, in the monistic sense, as the direct action of the material substratum, or whether we take matter and force to be distinct things in the dualistic sense, it is certain that we have not as yet found any living organism in which the exercise of the vital functions is not inseparably bound up with plasm.
The soft slimy plasson of the body of the moneron is generally called “protoplasm,” and identified with the cellular matter of the ordinary plant and animal cells. But we must, to be accurate, distinguish between the plasson of the cytodes and the protoplasm of the cells. This distinction is of the utmost importance for the purposes of evolution. As I have often said, we must recognise two different stages of development in these “elementary organisms,” or plastids (“builders”), that represent the ultimate units of organic individuality. The earlier and lower stage are the unnucleated cytodes, the body of which consists of only one kind of albuminous matter—the homogeneous plasson or “formative matter.” The later and higher stage are the nucleated cells, in which we find a differentiation of the original plasson into two different formative substances—the caryoplasm of the nucleus and the cytoplasm of the body of the cell (cf. pp. 37 and 42).

Fig. 226—Chroococcus minor (Nägeli), magnified. A phytomoneron, the globular plastids of which secrete a gelatinous structureless membrane. The unnucleated globule of plasm (bluish-green in colour) increases by simple cleavage (a–d).
The Monera are permanent cytodes. Their whole body consists of soft, structureless plasson. However carefully we examine it with our finest chemical reagents and most powerful microscopes, we can find no definite parts or no anatomic structure in it. Hence, the Monera are literally organisms without organs; in fact, from the philosophic point of view they are not organisms at all, since they have no organs. They can only be called organisms in the sense that they are capable of the vital functions of nutrition, reproduction, sensation, and movement. If we were to try to imagine the simplest possible organism, we should frame something like the moneron.
The Monera that we find to-day in various forms fall into two groups according to the nature of their nutrition—the Phytomonera and the Zoomonera; from the physiological point of view, the former are the simplest specimens of the plant (phyton) kingdom, and the latter of the animal (zoon) world. The Phytomonera, especially in their simplest form, the Chromacea (Phycochromacea or Cyanophycea), are the most primitive and the oldest of living organisms. The typical genus Chroococcus (Fig. 226) is represented by several fresh-water species, and often forms a very delicate bluish-green deposit on stones and wood in ponds and ditches. It consists of round, light green particles, from 1/7000 to 1/2500 of an inch in diameter.
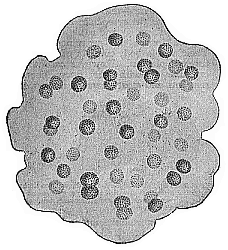
Fig. 227—Aphanocapsa primordialis (Nägeli), magnified. A phytomoneron, the round plastids of which (bluish-green in colour) secrete a shapeless gelatinous mass; in this the unnucleated cytodes increase continually by simple cleavage.
The whole life of these homogeneous globules of plasm consists of simple growth and reproduction by cleavage. When the tiny particle has reached a certain size by the continuous assimilation of inorganic matter, it divides into two equal halves, by a constriction in the middle. The two daughter-monera that are thus formed immediately begin a similar vital process. It is the same with the brown Procytella primordialis (formerly called the Protococcus marinus); it forms large masses of floating matter in the arctic seas. The tiny plasma-globules of this species are of a greenish-brown colour, and have a diameter of 1/10,000 to 1/5000 of an inch. There is no membrane discoverable in the simplest Chroococcacea, but we find one in other members of the same family; in Aphanocapsa (Fig. 227) the enveloping membranes of the social plastids combine; in Glœcapsa they are retained through several generations, so that the little plasma-globules are enfolded in many layers of membrane.
Next to the Chromacea come the Bacteria, which have been evolved from them by the remarkable change in nutrition which gives us the simple explanation of the differentiation of plant and animal in the protist kingdom. The Chromacea build up their plasm directly from inorganic matter; the Bacteria feed on organic matter. Hence, if we logically divide the protist kingdom into plasma-forming Protophyta and plasma-consuming Protozoa, we must class the Bacteria with the latter; it is quite illogical to describe them—as is still often done—as Schizomycetes, and class them with the true fungi. The Bacteria, like the Chromacea, have no nucleus. As is well-known, they play an important part in modern biology as the causes of fermentation and putrefaction, and of tuberculosis, typhus, cholera, and other infectious diseases, and as parasites, etc. But we cannot linger now to deal with these very interesting features; the Bacteria have no relation to man’s genealogical tree.
We may now turn to consider the remarkable Protamœba, or unnucleated Amœba. I have, in the first volume, pointed out the great importance of the ordinary Amœba in connection with several weighty questions of general biology. The tiny Protamœbæ, which are found both in fresh and salt water, have the same unshapely form and irregular movements of their simple naked body as the real Amœbæ; but they differ from them very materially in having no nucleus in their cell-body. The short, blunt, finger-like processes that are thrust out at the surface of the creeping Protamœba serve for getting food as well as for locomotion. They multiply by simple cleavage (Fig. 228).
The next stage to the simple cytode-forms of the Monera in the genealogy of mankind (and all other animals) is the simple cell, or the most rudimentary form of the cell which we find living independently to-day as the Amœba. The earliest process of inorganic differentiation in the structureless body of the Monera led to its division into two different substances—the caryoplasm and the cytoplasm. The caryoplasm is the inner and firmer part of the cell, the substance of the nucleus. The cytoplasm is the outer and softer part, the substance of the body of the cell. By this important differentiation of the plasson into nucleus and cell-body, the organised cell was evolved from the structureless cytode, the nucleated from the unnucleated plastid. That the first cells to appear on the earth were formed from the Monera by such a differentiation seems to us the only possible view in the present condition of science. We have a direct instance of this earliest process of differentiation to-day in the ontogeny of many of the lower Protists (such as the Gregarinæ).

Fig. 228—A moneron (Protamœba) in the act of reproduction. A The whole moneron, moving like an ordinary amœba by thrusting out changeable processes. B It divides into two halves by a constriction in the middle. C The two halves separate, and each becomes an independent individual. (Highly magnified.)
The unicellular form that we have in the ovum has already been described as the reproduction of a corresponding unicellular stem-form, and to this we have ascribed the organisation of an Amœba (cf. Chapter VI). The irregular-shaped Amœba, which we find living independently to-day in our fresh and salt water, is the least definite and the most primitive of all the unicellular Protozoa (Fig. 16). As the unripe ova (the protova that we find in the ovaries of animals) cannot be distinguished from the common Amœbæ, we must regard the Amœba as the primitive form that is reproduced in the embryonic stage of the amœboid ovum to-day, in accordance with the biogenetic law. I have already pointed out, in proof of the striking resemblance of the two cells, that the ova of many of the sponges were formerly regarded as parasitic Amœbæ (Figure 1.18). Large unicellular organisms like the Amœbæ were found creeping about inside the body of the sponge, and were thought to be parasites. It was afterwards discovered that they were really the ova of the sponge from which the embryos were developed. As a matter of fact, these sponge-ova are so much like many of the Amœbæ in size, shape, the character of their nucleus, and movement of the pseudopodia, that it is impossible to distinguish them without knowing their subsequent development.
Our phylogenetic interpretation of the ovum, and the reduction of it to some ancient amœboid ancestral form, supply the answer to the old problem: “Which was first, the egg or the chick?” We can now give a very plain answer to this riddle, with which our opponents have often tried to drive us into a corner. The egg came a long time before the chick. We do not mean, of course, that the egg existed from the first as a bird’s egg, but as an indifferent amœboid cell of the simplest character. The egg lived for thousands of years as an independent unicellular organism, the Amœba. The egg, in the modern physiological sense of the word, did not make its appearance until the descendants of the unicellular Protozoon had developed into multicellular animals, and these had undergone sexual differentiation. Even then the egg was first a gastræa-egg, then a platode-egg, then a vermalia-egg, and chordonia-egg; later still acrania-egg, then fish-egg, amphibia-egg, reptile-egg, and finally bird’s egg. The bird’s egg we have experience of daily is a highly complicated historical product, the result of countless hereditary processes that have taken place in the course of millions of years.
The earliest ancestors of our race were simple Protophyta, and from these our protozoic ancestors were developed afterwards. From the morphological point of view both the vegetal and the animal Protists were simple organisms, individualities of the first order, or plastids. All our later ancestors are complex organisms, or individualities of a higher order—social aggregations of a plurality of cells. The earliest of these, the Moræada, which represent the third stage in our genealogy, are very simple associations of homogeneous, indifferent cells—undifferentiated colonies of social Amœbæ or Infusoria. To understand the nature and origin of these protozoa-colonies we need only follow step by step the first embryonic products of the stem-cell. In all the Metazoa the first embryonic process is the repeated cleavage of the stem-cell, or first segmentation-cell (Fig. 229). We have already fully considered this process, and found that all the different forms of it may be reduced to one type, the original equal or primordial segmentation (cf. Chapter VIII). In the genealogical tree of the Vertebrates this palingenetic form of segmentation has been preserved in the Amphioxus alone, all the other Vertebrates having cenogenetically modified forms of cleavage. In any case, the latter were developed from the former, and so the segmentation of the ovum in the Amphioxus has a great interest for us (cf. Fig. 38). The outcome of this repeated cleavage is the formation of a round cluster of cells, composed of homogeneous, indifferent cells of the simplest character (Fig. 230). This is called the morula (= mulberry-embryo) on account of its resemblance to a mulberry or blackberry.
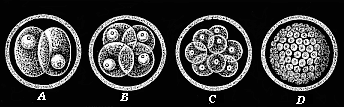
Fig. 229—Original or primordial ovum-cleavage. The stem-cell or cytula, formed by fecundation of the ovum, divides by repeated regular cleavage first into two (A), then four (B), then eight (C), and finally a large number of segmentation-cells (D).
It is clear that this morula reproduces for us to-day the simple structure of the multicellular animal that succeeded the unicellular amœboid form in the early Laurentian period. In accordance with the biogenetic law, the morula recalls the ancestral form of the Moræa, or simple colony of Protozoa. The first cell-communities to be formed, which laid the early foundation of the higher multicellular body, must have consisted of homogeneous and simple amœboid cells. The oldest Amœbæ lived isolated lives, and even the amœboid cells that were formed by the segmentation of these unicellular organisms must have continued to live independently for a long time. But gradually small communities of Amœbæ arose by the side of these eremitical Protozoa, the sister-cells produced by cleavage remaining joined together. The advantages in the struggle for life which these communities had over the isolated cells favoured their formation and their further development. We find plenty of these cell-colonies or communities to-day in both fresh and salt water. They belong to various groups both of the Protophyta and Protozoa.
To have some idea of those ancestors of our race that succeeded phylogenetically to the Moræada, we have only to follow the further embryonic development of the morula. We then see that the social cells of the round cluster secrete a sort of jelly or a watery fluid inside their globular body, and they themselves rise to the surface of it (Fig. 29 F, G). In this way the solid mulberry-embryo becomes a hollow sphere, the wall of which is composed of a single layer of cells. We call this layer the blastoderm, and the sphere itself the blastula, or embryonic vesicle.
This interesting blastula is very important. The conversion of the morula into a hollow ball proceeds on the same lines originally in the most diverse stems—as, for instance, in many of the zoophytes and worms, the ascidia, many of the echinoderms and molluscs, and in the amphioxus. Moreover, in the animals in which we do not find a real palingenetic blastula the defect is clearly due to cenogenetic causes, such as the formation of food-yelk and other embryonic adaptations. We may, therefore, conclude that the ontogenetic blastula is the reproduction of a very early phylogenetic ancestral form, and that all the Metazoa are descended from a common stem-form, which was in the main constructed like the blastula. In many of the lower animals the blastula is not developed within the fœtal membranes, but in the open water. In those cases each blastodermic cell begins at an early stage to thrust out one or more mobile hair-like processes; the body swims about by the vibratory movement of these lashes or whips (Fig. 29 F).
We still find, both in the sea and in fresh water, various kinds of primitive multicellular organisms that substantially resemble the blastula in structure, and may be regarded in a sense as permanent blastula-forms—hollow vesicles or gelatinous balls, with a wall composed of a single layer of ciliated homogeneous cells. There are “blastæads” of this kind even among the Protophyta—the familiar Volvocina, formerly classed with the infusoria. The common Volvox globator is found in the ponds in the spring—a small, green, gelatinous globule, swimming about by means of the stroke of its lashes, which rise in pairs from the cells on its surface. In the similar Halosphæra viridis also, which we find in the marine plancton (floating matter), a number of green cells form a simple layer at the surface of the gelatinous ball; but in this case there are no cilia.
Some of the infusoria of the flagellata-class (Signura, Magosphæra, etc.) are similar in structure to these vegetal clusters, but differ in their animal nutrition; they form the special group of the Catallacta. In September, 1869, I studied the development of one of these graceful animals on the island of Gis-Oe, off the coast of Norway (Magosphæra planula), Figures 2.231 and 2.232). The fully-formed body is a gelatinous ball, with its wall composed of thirty-two to sixty-four ciliated cells; it swims about freely in the sea. After reaching maturity the community is dissolved. Each cell then lives independently for some time, grows, and changes into a creeping amœba. This afterwards contracts, and clothes itself with a structureless membrane. The cell then looks just like an ordinary animal ovum. When it has been in this condition for some time the cell divides into two, four, eight, sixteen, thirty-two, and sixty-four cells. These arrange themselves in a round vesicle, thrust out vibratory lashes, burst the capsule, and swim about in the same magosphæra-form with which we started. This completes the life-circle of the remarkable and instructive animal.
If we compare these permanent blastulæ with the free-swimming ciliated larvæ or blastulæ, with similar construction, of many of the lower animals, we can confidently deduce from them that there was a very early and long-extinct common stem-form of substantially the same structure as the blastula. We may call it the Blastæa. Its body consisted, when fully formed, of a simple hollow ball, filled with fluid or structureless jelly, with a wall composed of a single stratum of ciliated cells. There were probably many genera and species of these blastæads in the Laurentian period, forming a special class of marine protists.
It is an interesting fact that in the plant kingdom also the simple hollow sphere is found to be an elementary form of the multicellular organism. At the surface and below the surface (down to a depth of 2000 yards) of the sea there are green globules swimming about, with a wall composed of a single layer of chlorophyll-bearing cells. The botanist Schmitz gave them the name of Halosphæra viridis in 1879.
The next stage to the Blastæa, and the sixth in our genealogical tree, is the Gastræa that is developed from it. As we have already seen, this ancestral form is particularly important. That it once existed is proved with certainty by the gastrula, which we find temporarily in the ontogenesis of all the Metazoa (Fig. 29 J, K). As we saw, the original, palingenetic form of the gastrula is a round or oval uni-axial body, the simple cavity of which (the primitive gut) has an aperture at one pole of its axis (the primitive mouth). The wall of the gut consists of two strata of cells, and these are the primary germinal layers, the animal skin-layer (ectoderm) and vegetal gut-layer (entoderm).
The actual ontogenetic development of the gastrula from the blastula furnishes sound evidence as to the phylogenetic origin of the Gastræa from the Blastæa. A pit-shaped depression appears at one side of the spherical blastula (Fig. 29 H). In the end this invagination goes so far that the outer or invaginated part of the blastoderm lies close on the inner or non-invaginated part (Fig. 29 J). In explaining the phylogenetic origin of the gastræa in the light of this ontogenetic process, we may assume that the one-layered cell-community of the blastæa began to take in food more largely at one particular part of its surface. Natural selection would gradually lead to the formation of a depression or pit at this alimentary spot on the surface of the ball. The depression would grow deeper and deeper. In time the vegetal function of taking in and digesting food would be confined to the cells that lined this hole; the other cells would see to the animal functions of locomotion, sensation, and protection. This was the first division of labour among the originally homogeneous cells of the blastæa.
Fig. 231—The Norwegian Magosphæra planula,
swimming about by means of the lashes or cilia at its surface.
Fig.
232—Section of same, showing how the pear-shaped cells in the
centre of the gelatinous ball are connected by a fibrous process. Each cell has
a contractile vacuole as well as a nucleus.
The effect, then, of this earliest histological differentiation was to produce two different kinds of cells—nutritive cells in the depression and locomotive cells on the surface outside. But this involved the severance of the two primary germinal layers—a most important process. When we remember that even man’s body, with all its various parts, and the body of all the other higher animals, are built up originally out of these two simple layers, we cannot lay too much stress on the phylogenetic significance of this gastrulation. In the simple primitive gut or gastric cavity of the gastrula and its rudimentary mouth we have the first real organ of the animal frame in the morphological sense; all the other organs were developed afterwards from these. In reality, the whole body of the gastrula is merely a “primitive gut.” I have shown already (Chapters VIII and XIX) that the two-layered embryos of all the Metazoa can be reduced to this typical gastrula. This important fact justifies us in concluding, in accordance with the biogenetic law, that their ancestors also were phylogenetically developed from a similar stem-form. This ancient stem-form is the gastræa.
The gastræa probably lived in the sea during the Laurentian period, swimming about in the water by means of its ciliary coat much as free ciliated gastrulæ do to-day. Probably it differed from the existing gastrula only in one essential point, though extinct millions of years ago. We have reason, from comparative anatomy and ontogeny, to believe that it multiplied by sexual generation, not merely asexually (by cleavage, gemmation, and spores), as was no doubt the case with the earlier ancestors. Some of the cells of the primary germ-layers probably became ova and others fertilising sperm. We base these hypotheses on the fact that we do to-day find the simplest form of sexual reproduction in some of the living gastræads and other lower animals, especially the sponges.
The fact that there are still in existence various kinds of gastræads, or lower Metazoa with an organisation little higher than that of the hypothetical gastræa, is a strong point in favour of our theory. There are not very many species of these living gastræads; but their morphological and phylogenetic interest is so great, and their intermediate position between the Protozoa and Metazoa so instructive, that I proposed long ago (1876) to make a special class of them. I distinguished three orders in this class—the Gastremaria, Physemaria, and Cyemaria (or Dicyemida). But we might also regard these three orders as so many independent classes in a primitive gastræad stem.
The Gastremaria and Cyemaria, the chief of these living gastræads, are small Metazoa that live parasitically inside other Metazoa, and are, as a rule, 1/50 to 1/25 of an inch long, often much less (Fig. 233, 1–15). Their soft body, devoid of skeleton, consists of two simple strata of cells, the primary germinal layers; the outer of these is thickly clothed with long hair-like lashes, by which the parasites swim about in the various cavities of their host. The inner germinal layer furnishes the sexual products. The pure type of the original gastrula (or archigastrula, Fig. 29 I) is seen in the Pemmatodiscus gastrulaceus, which Monticelli discovered in the umbrella of a large medusa (Pilema pulmo) in 1895; the convex surface of this gelatinous umbrella was covered with numbers of clear vesicles, of 1/25 to 1/8 inch in diameter, in the fluid contents of which the little parasites were swimming. The cup-shaped body of the Pemmatodiscus (Fig. 233, 1) is sometimes rather flat, and shaped like a hat or cone, at other times almost curved into a semi-circle. The simple hollow of the cup, the primitive gut (g), has a narrow opening (o). The skin layer (e) consists of long slender cylindrical cells, which bear long vibratory hairs; it is separated by a thin structureless, gelatinous plate (f) from the visceral or gut layer (i), the prismatic cells of which are much smaller and have no cilia. Pemmatodiscus propagates asexually, by simple longitudinal cleavage; on this account it has recently been regarded as the representative of a special order of gastræads (Mesogastria).
Probably a near relative of the Pemmatodiscus is the Kunstleria Gruveli (Fig. 233, 2). It lives in the body-cavity of Vermalia (Sipunculida), and differs from the former in having no lashes either on the large ectodermic cells (e) or the small entodermic (i); the germinal layers are separated by a thick, cup-shaped, gelatinous mass, which has been called the “clear vesicle” (f). The primitive mouth is surrounded by a dark ring that bears very strong and long vibratory lashes, and effects the swimming movements.
Pemmatodiscus and Kunstleria may be included in the family of the Gastremaria. To these gastræads with open gut are closely related the Orthonectida (Rhopalura, Fig. 233, 3–5). They live parasitically in the body-cavity of echinoderms (Ophiura) and vermalia; they are distinguished by the fact that their primitive gut-cavity is not empty, but filled with entodermic cells, from which the sexual cells are developed. These gastræads are of both sexes, the male (Fig. 3) being smaller and of a somewhat different shape from the oval female (Fig. 4).
The somewhat similar Dicyemida (Fig. 6) are distinguished from the preceding by the fact that their primitive gut-cavity is occupied by a single large entodermic cell instead of a crowded group of sexual cells. This cell does not yield sexual products, but afterwards divides into a number of cells (spores), each of which, without being impregnated, grows into a small embryo. The Dicyemida live parasitically in the body-cavity, especially the renal cavities, of the cuttle-fishes. They fall in several genera, some of which are characterised by the possession of special polar cells; the body is sometimes roundish, oval, or club-shaped, at other times long and cylindrical. The genus Conocyema (Figs. 7–15) differs from the ordinary Dicyema in having four polar pimples in the form of a cross, which may be incipient tentacles.
The classification of the Cyemaria is much disputed; sometimes they are held to be parasitic infusoria (like the Opalina), sometimes platodes or vermalia, related to the suctorial worms or rotifers, but having degenerated through parasitism. I adhere to the phylogenetically important theory that I advanced in 1876, that we have here real gastræads, primitive survivors of the common stem-group of all the Metazoa. In the struggle for life they have found shelter in the body-cavity of other animals.
The small Cœlenteria attached to the floor of the sea that I have called the Physemaria (Haliphysema and Gastrophysema) probably form a third order (or class) of the living gastræads. The genus Haliphysema (Figs. 234, 235) is externally very similar to a large rhizopod (described by the same name in 1862) of the family of the Rhabdamminida, which was at first taken for a sponge. In order to avoid confusion with these, I afterwards gave them the name of Prophysema. The whole mature body of the Prophysema is a simple cylindrical or oval tube, with a two-layered wall. The hollow of the tube is the gastric cavity, and the upper opening of it the mouth (Fig. 235 m).
Fig. 233—Modern gastræads. Fig. 1. Pemmatodiscus gastrulaceus (Monticelli), in longitudinal section. Fig. 2. Kunstleria gruveli (Delage), in longitudinal section. (From Kunstler and Gruvel.) Figs. 3–5. Rhopalura Giardi (Julin): Fig. 3 male, Fig. 4 female, Fig. 5 planula. Fig. 6. Dicyema macrocephala (Van Beneden). Figs. 7–15. Conocyema polymorpha (Van Beneden): Fig. 7 the mature gastræad, Figs. 8–15 its gastrulation. d primitive gut, o primitive mouth, e ectoderm, i entoderm, f gelatinous plate between e and i (supporting plate, blastocœl).
The two strata of cells that form the wall of the tube are the primary germinal layers. These rudimentary zoophytes differ from the swimming gastræads chiefly in being attached at one end (the end opposite to the mouth) to the floor of the sea.
Figs. 234 and 235—Prophysema primordiale, a living gastræad. Fig. 234. The whole of the spindle-shaped animal (attached below to the floor of the sea. Fig. 235. The same in longitudinal section. The primitive gut (d) opens above at the primitive mouth (m). Between the ciliated cells (g) are the amœboid ova (e). The skin-layer (h) is encrusted with grains of sand below and sponge-spicules above.
In Prophysema the primitive gut is a simple oval cavity, but in the closely related Gastrophysema it is divided into two chambers by a transverse constriction; the hind and smaller chamber above furnishes the sexual products, the anterior one being for digestion.
Figs. 236–237—Ascula of gastrophysema, attached to the floor of the sea. Fig. 236 external view, 237 longitudinal section. g primitive gut, o primitive mouth, i visceral layer, e cutaneous layer. (Diagram.)
The simplest sponges (Olynthus, Fig. 238) have the same organisation as the Physemaria. The only material difference between them is that in the sponge the thin two-layered body-wall is pierced by numbers of pores. When these are closed they resemble the Physemaria. Possibly the gastræads that we call Physemaria are only olynthi with the pores closed. The Ammoconida, or the simple tubular sand-sponges of the deep-sea (Ammolynthus, etc.), do not differ from the gastræads in any important point when the pores are closed. In my Monograph on the Sponges (with sixty plates) I endeavoured to prove analytically that all the species of this class can be traced phylogenetically to a common stem-form (Calcolynthus).
The lowest form of the Cnidaria is also not far removed from the gastræads. In the interesting common fresh-water polyp (Hydra) the whole body is simply an oval tube with a double wall; only in this case the mouth has a crown of tentacles. Before these develop the hydra resembles an ascula (Figs. 236, 237). Afterwards there are slight histological differentiations in its ectoderm, though the entoderm remains a single stratum of cells. We find the first differentiation of epithelial and stinging cells, or of muscular and neural cells, in the thick ectoderm of the hydra.
In all these rudimentary living cœlenteria the sexual cells of both kinds—ova and sperm cells—are formed by the same individual; it is possible that the oldest gastræads were hermaphroditic. It is clear from comparative anatomy that hermaphrodism—the combination of both kinds of sexual cells in one individual—is the earliest form of sexual differentiation; the separation of the sexes (gonochorism) was a much later phenomenon. The sexual cells originally proceeded from the edge of the primitive mouth of the gastræad.
The gastræa theory has now convinced us that all the Metazoa or multicellular animals can be traced to a common stem-form, the Gastræa. In accordance with the biogenetic law, we find solid proof of this in the fact that the two-layered embryos of all the Metazoa can be reduced to a primitive common type, the gastrula. Just as the countless species of the Metazoa do actually develop in the individual from the simple embryonic form of the gastrula, so they have all descended in past time from the common stem-form of the Gastræa. In this fact, and the fact we have already established that the Gastræa has been evolved from the hollow vesicle of the one-layered Blastæa, and this again from the original unicellular stem-form, we have obtained a solid basis for our study of evolution. The clear path from the stem-cell to the gastrula represents the first section of our human stem-history (Chapters VIII, IX, and XIX).
The second section, that leads from the Gastræa to the Prochordonia, is much more difficult and obscure. By the Prochordonia we mean the ancient and long-extinct animals which the important embryonic form of the chordula proves to have once existed (cf. Figs. 83–86). The nearest of living animals to this embryonic structure are the lowest Tunicates, the Copelata ( Appendicaria) and the larvæ of the Ascidia. As both the Tunicates and the Vertebrates develop from the same chordula, we may infer that there was a corresponding common ancestor of both stems. We may call this the Chordæa, and the corresponding stem-group the Prochordonia or Prochordata.
From this important stem-group of the unarticulated Prochordonia (or “primitive chorda-animals”) the stems of the Tunicates and Vertebrates have been divergently evolved. We shall see presently how this conclusion is justified in the present condition of morphological science.
We have first to answer the difficult and much-discussed question of the development of the Chordæa from the Gastræa; in other words, “How and by what transformations were the characteristic animals, resembling the embryonic chordula, which we regard as the common stem-forms of all the Chordonia, both Tunicates and Vertebrates, evolved from the simplest two-layered Metazoa?”
The descent of the Vertebrates from the Articulates has been maintained by a number of zoologists during the last thirty years with more zeal than discernment; and, as a vast amount has been written on the subject, we must deal with it to some extent. All three classes of Articulates in succession have been awarded the honour of being considered the “real ancestors” of the Vertebrates: first, the Annelids (earth-worms, leeches, and the like), then the Crustacea (crabs, etc.), and, finally, the Tracheata (spiders, insects, etc.). The most popular of these hypotheses was the annelid theory, which derived the Vertebrates from the Worms. It was almost simultaneously (1875) formulated by Carl Semper, of Würtzburg, and Anton Dohrn, of Naples. The latter advanced this theory originally in favour of the failing degeneration theory, with which I dealt in my work, Aims and Methods of Modern Embryology.
This interesting degeneration theory—much discussed at that time, but almost forgotten now—was formed in 1875 with the aim of harmonising the results of evolution and ever-advancing Darwinism with religious belief. The spirited struggle that Darwin had occasioned by the reformation of the theory of descent in 1859, and that lasted for a decade with varying fortunes in every branch of biology, was drawing to a close in 1870–1872, and soon ended in the complete victory of transformism. To most of the disputants the chief point was not the general question of evolution, but the particular one of “man’s place in nature”—“the question of questions,” as Huxley rightly called it. It was soon evident to every clear-headed thinker that this question could only be answered in the sense of our anthropogeny, by admitting that man had descended from a long series of Vertebrates by gradual modification and improvement.
In this way the real affinity of man and the Vertebrates came to be admitted on all hands. Comparative anatomy and ontogeny spoke too clearly for their testimony to be ignored any longer. But in order still to save man’s unique position, and especially the dogma of personal immortality, a number of natural philosophers and theologians discovered an admirable way of escape in the “theory of degeneration.” Granting the affinity, they turned the whole evolutionary theory upside down, and boldly contended that “man is not the most highly developed animal, but the animals are degenerate men.” It is true that man is closely related to the ape, and belongs to the vertebrate stem; but the chain of his ancestry goes upward instead of downward. In the beginning “God created man in his own image,” as the prototype of the perfect vertebrate; but, in consequence of original sin, the human race sank so low that the apes branched off from it, and afterwards the lower Vertebrates. When this theory of degeneration was consistently developed, its supporters were bound to hold that the entire animal kingdom was descended from the debased children of men.
This theory was most strenuously defended by the Catholic priest and natural philosopher, Michelis, in his Hæckelogony: An Academic Protest against Hæckel’s Anthropogeny (1875). In still more “academic” and somewhat mystic form the theory was advanced by a natural philosopher of the older Jena school—the mathematician and physicist, Carl Snell. But it received its chief support on the zoological side from Anton Dohrn, who maintained the anthropocentric ideas of Snell with particular ability. The Amphioxus, which modern science now almost unanimously regards as the real Primitive Vertebrate, the ancient model of the original vertebrate structure, is, according to Dohrn, a late, degenerate descendant of the stem, the “prodigal son” of the vertebrate family. It has descended from the Cyclostoma by a profound degeneration, and these in turn from the fishes; even the Ascidia and the whole of the Tunicates are merely degenerate fishes! Following out this curious theory, Dohrn came to contest the general belief that the Cœlenterata and Worms are “lower animals”; he even declared that the unicellular Protozoa were degenerate Cœlenterata. In his opinion “degeneration is the great principle that explains the existence of all the lower forms.”
If this Michelis-Dohrn theory were true, and all animals were really degenerate descendants of an originally perfect humanity, man would assuredly be the true centre and goal of all terrestrial life; his anthropocentric position and his immortality would be saved. Unfortunately, this trustful theory is in such flagrant contradiction to all the known facts of paleontology and embryology that it is no longer worth serious scientific consideration.
But the case is no better for the much-discussed descent of the Vertebrates from the Annelids, which Dohrn afterwards maintained with great zeal. Of late years this hypothesis, which raised so much dust and controversy, has been entirely abandoned by most competent zoologists, even those who once supported it. Its chief supporter, Dohrn, admitted in 1890 that it is “dead and buried,” and made a blushing retraction at the end of his Studies of the Early History of the Vertebrate.
Now that the annelid-hypothesis is “dead and buried,” and other attempts to derive the Vertebrates from Medusæ, Echinoderms, or Molluscs, have been equally unsuccessful, there is only one hypothesis left to answer the question of the origin of the Vertebrates—the hypothesis that I advanced thirty-six years ago and called the “chordonia-hypothesis.” In view of its sound establishment and its profound significance, it may very well claim to be a theory, and so should be described as the chordonia or chordæa theory.
I first advanced this theory in a series of university lectures in 1867, from which the History of Creation was composed. In the first edition of this work (1868) I endeavoured to prove, on the strength of Kowalevsky’s epoch-making discoveries, that “of all the animals known to us the Tunicates are undoubtedly the nearest blood-relatives of the Vertebrates; they are the most closely related to the Vermalia, from which the Vertebrates have been evolved. Naturally, I do not mean that the Vertebrates have descended from the Tunicates, but that the two groups have sprung from a common root. It is clear that the real Vertebrates (primarily the Acrania) were evolved in very early times from a group of Worms, from which the degenerate Tunicates also descended in another and retrogressive direction.” This common extinct stem-group are the Prochordonia; we still have a silhouette of them in the chordula-embryo of the Vertebrates and Tunicates; and they still exist independently, in very modified form, in the class of the Copelata ( Appendicaria, Fig. 225).
The chordæa-theory received the most valuable and competent support from Carl Gegenbaur. This able comparative morphologist defended it in 1870, in the second edition of his Elements of Comparative Anatomy ; at the same time he drew attention to the important relations of the Tunicates to a curious worm, Balanoglossus : he rightly regards this as the representative of a special class of worms, which he called “gut-breathers” ( Enteropneusta). Gegenbaur referred on many other occasions to the close blood-relationship of the Tunicates and Vertebrates, and luminously explained the reasons that justify us in framing the hypothesis of the descent of the two stems from a common ancestor, an unsegmented worm-like animal with an axial chorda between the dorsal nerve-tube and the ventral gut-tube.
The theory afterwards received a good deal of support from the research made by a number of distinguished zoologists and anatomists, especially C. Kupffer, B. Hatschek, F. Balfour, E. Van Beneden, and Julin. Since Hatschek’s Studies of the Development of the Amphioxus gave us full information as to the embryology of this lowest vertebrate, it has become so important for our purpose that we must consider it a document of the first rank for answering the question we are dealing with.
The ontogenetic facts that we gather from this sole survivor of the Acrania are the more valuable for phylogenetic purposes, as paleontology, unfortunately, throws no light whatever on the origin of the Vertebrates. Their invertebrate ancestors were soft organisms without skeleton, and thus incapable of fossilisation, as is still the case with the lowest vertebrates—the Acrania and Cyclostoma. The same applies to the greater part of the Vermalia or worm-like animals, the various classes and orders of which differ so much in structure. The isolated groups of this rich stem are living branches of a huge tree, the greater part of which has long been dead, and we have no fossil evidence as to its earlier form. Nevertheless, some of the surviving groups are very instructive, and give us clear indications of the way in which the Chordonia were developed from the Vermalia, and these from the Cœlenteria.
While we seek the most important of these palingenetic forms among the groups of Cœlenteria and Vermalia, it is understood that not a single one of them must be regarded as an unchanged, or even little changed, copy of the extinct stem-form. One group has retained one feature, another a different feature, of the original organisation, and other organs have been further developed and characteristically modified. Hence here, more than in any other part of our genealogical tree, we have to keep before our mind the full picture of development, and separate the unessential secondary phenomena from the essential and primary. It will be useful first to point out the chief advances in organisation by which the simple Gastræa gradually became the more developed Chordæa.
We find our first solid datum in the gastrula of the Amphioxus (Figure 1.38). Its bilateral and tri-axial type indicates that the Gastræads—the common ancestors of all the Metazoa—divided at an early stage into two divergent groups. The uni-axial Gastræa became sessile, and gave rise to two stems, the Sponges and the Cnidaria (the latter all reducible to simple polyps like the hydra). But the tri-axial Gastræa assumed a certain pose or direction of the body on account of its swimming or creeping movement, and in order to sustain this it was a great advantage to share the burden equally between the two halves of the body (right and left). Thus arose the typical bilateral form, which has three axes. The same bilateral type is found in all our artificial means of locomotion—carts, ships, etc.; it is by far the best for the movement of the body in a certain direction and steady position. Hence natural selection early developed this bilateral type in a section of the Gastræads, and thus produced the stem-forms of all the bilateral animals.
The Gastræa bilateralis, of which we may conceive the bilateral gastrula of the amphioxus to be a palingenetic reproduction, represented the two-sided organism of the earliest Metazoa in its simplest form. The vegetal entoderm that lined their simple gut-cavity served for nutrition; the ciliated ectoderm that formed the external skin attended to locomotion and sensation; finally, the two primitive mesodermic cells, that lay to the right and left at the ventral border of the primitive mouth, were sexual cells, and effected reproduction. In order to understand the further development of the gastræa, we must pay particular attention to: (1) the careful study of the embryonic stages of the amphioxus that lie between the gastrula and the chordula; (2) the morphological study of the simplest Platodes ( Platodaria and Turbellaria) and several groups of unarticulated Vermalia ( Gastrotricha, Nemertina, Enteropneusta).
We have to consider the Platodes first, because they are on the border between the two principal groups of the Metazoa, the Cœlenteria and the Cœlomaria. With the former they share the lack of body-cavity, anus, and vascular system; with the latter they have in common the bilateral type, the possession of a pair of nephridia or renal canals, and the formation of a vertical brain or cerebral ganglion. It is now usual to distinguish four classes of Platodes: the two free-living classes of the primitive worms ( Platodaria) and the coiled-worms ( Turbellaria), and the two parasitic classes of the suctorial worms ( Trematoda) and the tape-worms ( Cestoda). We have only to consider the first two of these classes; the other two are parasites, and have descended from the former by adaptation to parasitic habits and consequent degeneration.
The primitive worms ( Platodaria) are very small flat worms of simple construction, but of great morphological and phylogenetic interest. They have been hitherto, as a rule, regarded as a special order of the Turbellaria, and associated with the Rhabdocœla ; but they differ considerably from these and all the other Platodes (flat worms) in the absence of renal canals and a special central nervous system; the structure of their tissue is also simpler than in the other Platodes. Most of the Platodes of this group ( Aphanostomum, Amphichœrus, Convoluta, Schizoprora, etc.) are very soft and delicate animals, swimming about in the sea by means of a ciliary coat, and very small (1/10 to 1/20 inch long). Their oval body, without appendages, is sometimes spindle-shaped or cylindrical, sometimes flat and leaf-shaped. Their skin is merely a layer of ciliated ectodermic cells. Under this is a soft medullary substance, which consists of entodermic cells with vacuoles. The food passes through the mouth directly into this digestive medullary substance, in which we do not generally see any permanent gut-cavity (it may have entirely collapsed); hence these primitive Platodes have been called Acœla (without gut-cavity or cœlom), or, more correctly, Cryptocœla, or Pseudocœla. The sexual organs of these hermaphroditic Platodaria are very simple—two pairs of strings of cells, the inner of which (the ovaries, Fig. 239 o) produce ova, and the outer (the spermaria, s) sperm-cells. These gonads are not yet independent sexual glands, but sexually differentiated cell-groups in the medullary substance, or, in other words, parts of the gut-wall. Their products, the sex-cells, are conveyed out behind by two pairs of short canals; the male opening ( m) lies just behind the female ( f). Most of the Platodaria have not the muscular pharynx, which is very advanced in the Turbellaria and Trematoda. On the other hand, they have, as a rule, before or behind the mouth, a bulbous sense-organ (auditory vesicle or organ of equilibrium, g), and many of them have also a couple of simple optic spots. The cell-pit of the ectoderm that lies underneath is rather thick, and represents the first rudiment of a neural ganglion (vertical brain or acroganglion).
Fig. 239—Aphanostomum Langii ( Haeckel), a primitive worm of the platodaria class, of the order of Cryptocoela or Acoela. This new species of the genus Aphanostomum, named after Professor Arnold Lang of Zurich, was found in September, 1899, at Ajaccio in Corsica (creeping between fucoidea). It is one-twelfth of an inch long, one-twenty-fifth of an inch broad, and violet in colour. a mouth, g auditory vesicle, e ectoderm, i entoderm, o ovaries, a spermaries, f female aperture, m male aperture.
The Turbellaria, with which the similar Platodaria were formerly classed, differ materially from them in the more advanced structure of their organs, and especially in having a central nervous system (vertical brain) and excretory renal canals (nephridia); both originate from the ectoderm. But between the two germinal layers a mesoderm is developed, a soft mass of connective tissue, in which the organs are embedded. The Turbellaria are still represented by a number of different forms, in both fresh and sea-water. The oldest of these are the very rudimentary and tiny forms that are known as Rhabdocœla on account of the simple construction of their gut; they are, as a rule, less than a quarter of an inch long and of a simple oval or lancet shape (Fig. 240). The surface is covered with ciliated epithelium, a stratum of ectodermic cells. The digestive gut is still the simple primitive gut of the gastræa ( d), with a single aperture that is both mouth and anus ( m). There is, however, an invagination of the ectoderm at the mouth, which has given rise to a muscular pharynx ( sd). It is noteworthy that the mouth of the Turbellaria (like the primitive mouth of the Gastræa) may, in this class, change its position considerably in the middle line of the ventral surface; sometimes it lies behind ( Opisthostomum), sometimes in the middle ( Mesostomum), sometimes in front ( Prosostomum). This displacement of the mouth from front to rear is very interesting, because it corresponds to a phylogenetic displacement of the mouth. This probably occurred in the Platode ancestors of most (or all?) of the Cœlomaria; in these the permanent mouth ( metastoma) lies at the fore end (oral pole), whereas the primitive mouth ( prostoma) lay at the hind end of the bilateral body.
In most of the Turbellaria there is a narrow cavity, containing a number of secondary organs, between the two primary germinal layers, the outer or animal layer of which forms the epidermis and the inner vegetal layer the visceral epithelium. The earliest of these organs are the sexual organs; they are very variously constructed in the Platode-class; in the simplest case there are merely two pairs of gonads or sexual glands—a pair of testicles (Fig. 241 h) and a pair of ovaries ( e). They open externally, sometimes by a common aperture ( Monogonopora), sometimes by separate ones, the female behind the male ( Digonopora, Fig. 241). The sexual glands develop originally from the two promesoblasts or primitive mesodermic cells (Fig. 83 p). As these earliest mesodermic structures extended, and became spacious sexual pouches in the later descendants of the Platodes, probably the two cœlom-pouches were formed from them, the first trace of the real body-cavity of the higher Metazoa ( Enterocœla).
The gonads are among the oldest organs, the few other organs that we find in the Platodes between the gut-wall and body-wall being later evolutionary products. One of the oldest and most important of these are the kidneys or nephridia, which remove unusable matter from the body (Fig. 240 nc). These urinary or excretory organs were originally enlarged skin-glands—a couple of canals that run the length of the body, and have a separate or common external aperture ( nm). They often have a number of branches. These special excretory organs are not found in the other Cœlenteria (Gastræads, Sponges, Cnidaria) or the Cryptocœla. They are first met in the Turbellaria, and have been transmitted direct from these to the Vermalia, and from these to the higher stems.
Fig. 240—A simple turbellarian (
Rhabdocœlum). m mouth, sd gullet epithelium, sm
gullet muscles, d gastric gut, nc renal canals, nm renal
aperture, au eye, na olfactory pit. (Diagram.)
Fig. 241—The same, showing the other organs. g brain,
au eye, na olfactory pit, n nerves, h testicles,
ma male aperture, fa female aperture, e ovary, f
ciliated epiderm. (Diagram.)
Finally, there is a very important new organ in the Turbellaria, which we do not find in the Cryptocœla (Fig. 239) and their gastræad ancestors—the rudimentary nervous system. It consists of a couple of simple cerebral ganglia (Fig. 241 g) and fine nervous fibres that radiate from them; these are partly voluntary nerves (or motor fibres) that go to the thin muscular layer developing under the skin; and partly sensory nerves that proceed to the sense-cells of the ciliated epiderm ( f). Many of the Turbellaria have also special sense-organs; a couple of ciliated smell pits ( na), rudimentary eyes ( au), and, less frequently, auditory vesicles.
On these principles I assume that the oldest and simplest Turbellaria arose from Platodaria, and these directly from bilateral Gastræads. The chief advances were the formation of gonads and nephridia, and of the rudimentary brain. On this hypothesis, which I advanced in 1872 in the first sketch of the gastræa-theory ( Monograph on the Sponges), there is no direct affinity between the Platodes and the Cnidaria.
Next to the ancient stem-group of the Turbellaria come a number of more recent chordonia ancestors, which we class with the Vermalia or Helminthes, the unarticulated worms. These true worms ( Vermes, lately also called Scolecida) are the difficulty or the lumber-room of the zoological classifier, because the various classes have very complicated relations to the lower Platodes on the one hand and the more advanced animals on the other. But if we exclude the Platodes and the Annelids from this stem, we find a fairly satisfactory unity of organisation in the remaining classes. Among these worms we find some important forms that show considerable advance in organisation from the platode to the chordonia stage. Three of these phenomena are particularly instructive: (1) The formation of a true (secondary) body-cavity (cœloma); (2) the formation of a second aperture of the gut, the anus; and (3) the formation of a vascular system. The great majority of the Vermalia have these three features, and they are all wanting in the Platodes; in the rest of the worms at least one or two of them are developed.
Figs. 242 and 243—Chætonotus, a rudimentary vermalian, of the group of Gastrotricha. m mouth, s gullet, d gut, a anus, g brain, n nerves, ss sensory hairs, au eye, ms muscular cells, h skin, f ciliated bands of the ventral surface, nc nephridia, nm their aperture, e ovaries.
Next and very close to the Platodes we have the Ichthydina ( Gastrotricha), little marine and fresh-water worms, about 1/250 to 1/1000 inch long. Zoologists differ as to their position in classification. In my opinion, they approach very close to the Rhabdocœla (Figs. 240, 241), and differ from them chiefly in the possession of an anus at the posterior end (Fig. 242 a). Further, the cilia that cover the whole surface of the Turbellaria are confined in the Gastrotricha to two ciliated bands ( f) on the ventral surface of the oval body, the dorsal surface having bristles. Otherwise the organisation of the two classes is the same. In both the gut consists of a muscular gullet ( s) and a glandular primitive gut ( d). Over the gullet is a double brain (acroganglion, g). At the side of the gut are two serpentine prorenal canals (water-vessels or pronephridia, nc), which open on the ventral side ( nm). Behind are a pair of simple sexual glands or gonads (Fig. 243 e).
While the Ichthydina are thus closely related to the Platodes, we have to go farther away for the two classes of Vermalia which we unite in the group of the “snout-worms” ( Frontonia). These are the Nemertina and the Enteropneusta. Both classes have a complete ciliary coat on the epidermis, a heritage from the Turbellaria and the Gastræads; also, both have two openings of the gut, the mouth and anus, like the Gastrotricha. But we find also an important organ that is wanting in the preceding forms—the vascular system. In their more advanced mesoderm we find a few contractile longitudinal canals which force the blood through the body by their contractions; these are the first blood-vessels.
Fig. 244—A simple Nemertine. m mouth, d gut, a anus, g brain, n nerves, h ciliary coat, ss sensory pits (head-clefts), au eyes, r dorsal vessel, l lateral vessels. (Diagram.)
Fig. 245—A young Enteropneust ( Balanaglossus). (From Alexander Agassiz.) r acorn-shaped snout, h neck, k gill-clefts and gill-arches of the fore-gut, in long rows on each side, d digestive hind-gut, filling the greater part of the body-cavity, v intestinal vein or ventral vessel, lying between the parallel folds of the skin, a anus.
The Nemertina were formerly classed with the much less advanced Turbellaria. But they differ essentially from them in having an anus and blood-vessels, and several other marks of higher organisation. They have generally long and narrow bodies, like a more or less flattened cord; there are, besides several small species, giant-forms with a width of 1/5 to 2/5 inch and a length of several yards (even ten to fifteen). Most of them live in the sea, but some in fresh water and moist earth. In their internal structure they approach the Turbellaria on the one hand and the higher Vermalia (especially the Enteropneusta) on the other. They have a good deal of interest as the lowest and oldest of all animals with blood. In them we find blood-vessels for the first time, distributing real blood through the body. The blood is red, and the red colouring-matter is hæmoglobin, connected with elliptic discoid blood-cells, as in the Vertebrates. Most of them have two or three parallel blood-canals, which run the whole length of the body, and are connected in front and behind by loops, and often by a number of ring-shaped pieces. The chief of these primitive blood-vessels is the one that lies above the gut in the middle line of the back (Fig. 244 r); it may be compared to either the dorsal vessel of the Articulates or the aorta of the Vertebrates. To the right and left are the two serpentine lateral vessels (Fig. 244 l).
Fig. 246—Transverse section of the branchial gut. A of Balanoglossus, B of Ascidia. r branchial gut, n pharyngeal groove, * ventral folds between the two. Diagrammatic illustration from Gegenbaur, to show the relation of the dorsal branchial-gut cavity ( r) to the pharyngeal or hypobranchial groove ( n).
After the Nemertina, I take (as distant relatives) the Enteropneusta ; they may be classed together with them as Frontonia or Rhyncocœla (snout-worms). There is now only one genus of this class, with several species ( Balanoglossus); but it is very remarkable, and may be regarded as the last survivor of an ancient and long-extinct class of Vermalia. They are related, on the one hand, to the Nemertina and their immediate ancestors, the Platodes, and to the lowest and oldest forms of the Chordonia on the other.
The Enteropneusta (Fig. 245) live in the sea sand, and are long worms of very simple shape, like the Nemertina. From the latter they have inherited: (1) The bilateral type, with incomplete segmentation; (2) the ciliary coat of the soft epidermis; (3) the double rows of gastric pouches, alternating with a single or double row of gonads; (4) separation of the sexes (the Platode ancestors were hermaphroditic); (5) the ventral mouth, underneath a protruding snout; (6) the anus terminating the simple gut-tube; and (7) several parallel blood-canals, running the length of the body, a dorsal and a ventral principal stem.
On the other hand, the Enteropneusta differ from their Nemertine ancestors in several features, some of which are important, that we may attribute to adaptation. The chief of these is the branchial gut (Fig. 245 k). The anterior section of the gut is converted into a respiratory organ, and pierced by two rows of gill-clefts; between these there is a branchial (gill) skeleton, formed of rods and plates of chitine. The water that enters at the mouth makes its exit by these clefts. They lie in the dorsal half of the fore-gut, and this is completely separated from the ventral half by two longitudinal folds (Fig. 246 A*). This ventral half, the glandular walls of which are clothed with ciliary epithelium and secrete mucus, corresponds to the pharyngeal or hypo-branchial groove of the Chordonia ( Bn), the important organ from which the later thyroid gland is developed in the Craniota (cf. p. 184). The agreement in the structure of the branchial gut of the Enteropneusts, Tunicates, and Vertebrates was first recognised by Gegenbaur (1878); it is the more significant as at first we find only a couple of gill-clefts in the young animals of all three groups; the number gradually increases. We can infer from this the common descent of the three groups with all the more confidence when we find the Balanoglossus approaching the Chordonia in other respects. Thus, for instance, the chief part of the central nervous system is a long dorsal neural string that runs above the gut and corresponds to the medullary tube of the Chordonia. Bateson believes he has detected a rudimentary chorda between the two.
Of all extant invertebrate animals the Enteropneusts come nearest to the Chordonia in virtue of these peculiar characters; hence we may regard them as the survivors of the ancient gut-breathing Vermalia from which the Chordonia also have descended. Again, of all the chorda-animals the Copelata (Fig. 225) and the tailed larvæ of the ascidia approach nearest to the young Balanoglossus. Both are, on the other hand, very closely related to the Amphioxus, the Primitive Vertebrate of which we have considered the importance (Chapters XVI and XVII). As we saw there, the unarticulated Tunicates and the articulated Vertebrates must be regarded as two independent stems, that have developed in divergent directions. But the common root of the two stems, the extinct group of the Prochordonia, must be sought in the vermalia stem; and of all the living Vermalia those we have considered give us the safest clue to their origin. It is true that the actual representatives of the important groups of the Copelata, Balanoglossi, Nemertina, Icthydina, etc., have more or less departed from the primitive model owing to adaptation to special environment. But we may just as confidently affirm that the main features of their organisation have been preserved by heredity.
We must grant, however, that in the whole stem-history of the Vertebrates the long stretch from the Gastræads and Platodes up to the oldest Chordonia remains by far the most obscure section. We might frame another hypothesis to raise the difficulty—namely, that there was a long series of very different and totally extinct forms between the Gastræa and the Chordæa. Even in this modified chordæa-theory the six fundamental organs of the chordula would retain their great value. The medullary tube would be originally a chemical sensory organ, a dorsal olfactory tube, taking in respiratory-water and food by the neuroporus in front and conveying them by the neurenteric canal into the primitive gut. This olfactory tube would afterwards become the nervous centre, while the expanding gonads (lying to right and left of the primitive mouth) would form the cœloma. The chorda may have been originally a digestive glandular groove in the dorsal middle line of the primitive gut. The two secondary gut-openings, mouth and anus, may have arisen in various ways by change of functions. In any case, we should ascribe the same high value to the chordula as we did before to the gastrula.
In order to explain more fully the chief stages in the advance of our race, I add the hypothetical sketch of man’s ancestry that I published in my Last Link [a translation by Dr. Gadow of the paper read at the International Zoological Congress at Cambridge in 1898]:—
A.—Man’s Genealogical Tree, First Half:
EARLIER SERIES OF ANCESTORS,
WITHOUT FOSSIL EVIDENCE.
| Chief Stages | Ancestral Stem-groups | Living Relatives of Ancestors |
| Stages 1–5: Protist ancestors Unicellular organisms. 1–2: Prototypes 3–5: Protozoa | 1.
Monera Without nucleus 2. Algaria Unicellular algæ | 1. Chromacea (Chroococcus) Phycochromacea 2. Paulotomea Palmellacea Eremosphæra |
| 3. Lobosa Unicellular (amœbina) rhizopods 4. Infusoria Unicellular 5. Blastæades Multicellular hollow spheres | 3. Amœbina Amœba Leucocyta 4. Flagellata Euflagellata Zoomonades 5. Catallacta Magosphæra, Volvocina, Blastula |
|
| Stages 6–11: Invertebrate metazoa ancestors 6–8: Cœlenteria without anus and body-cavity 9–11: Vermalia, with anus and body-cavity | 6.
Gastræades With two germ-layers 7 Platodes I Platodaria (without nephridia) 8. Platodes II Platodinia (with nephridia) | 6.
Gastrula Hydra, Olynthus, Gastremaria 7. Cryptocœla Convoluta, Porporus 8. Rhabdocœla Vortex, Monolus |
| 9. Provermalia (Primitive worms) Rotatoria 10. Frontonia (Rhynchelminthes) Snout-worms 11. Prochordonia Chorda-worms | 9. Gastrotricha Trochozoa, Trochophora 10. Enteropneusta Balanglossus Cephalodiscus 11. Copelata Appendicaria Chordula-larvæ | |
| Stages 12–15: Monorhina ancestors Oldest vertebrates without jaws or pairs of limbs, single nose | 12. Acrania I
(Prospondylia) 13. Acrania II More recent 14. Cyclostoma I (Archicrania) 15. Cyclostoma II More recent |
12. Amphioxus larvæ 13. Leptocardia Amphioxus 14. Petromyzonta larvæ 15. Marsipobranchia Petromyzonta |
B.—Man’s Genealogical Tree, Second Half:
LATER ANCESTORS, WITH FOSSIL EVIDENCE.
| Geological Periods | Ancestral Stem-groups | Living Relatives of Ancestors |
| Silurian |
16. Selachii Primitive fishes Proselachii | 16. Natidanides Chlamydoselachius Heptanchus |
| Silurian |
17. Ganoids Plated-fishes Proganoids | 17. Accipenserides (Sturgeons) Polypterus |
| Devonian | 18. Dipneusta Paladipneusta | 18. Neodipneusta Ceratodus Proptopterus |
| Carboniferous | 19. Amphibia Stegocephala | 19. Phanerobranchia Salamandrina (Proteus, triton) |
| Permian | 20. Reptilia Proreptilia | 20. Rhynchocephalia Primitive lizards Hatteria |
| Triassic | 21. Monotrema Promammalia | 21. Ornithodelphia Echidna Ornithorhyncus |
| Jurassic | 22. Marsupialia Prodidelphia | 22. Didelphia Didelphys Perameles |
| Cretaceous | 23. Mallotheria Prochoriata | 23. Insectivora Erinaceida (Ictopsia +) |
| Older Eocene | 24. Lemuravida Older lemurs Dentition 3. 1. 4. 3. | 24. Pachylemures (Hyopsodus +) (Adapis +) |
| Neo-Eocene |
25. Lemurogona Later lemurs Dentition 2. 1. 4. 3. | 25. Autolemures Eulemur Stenops |
| Oligocene |
26. Dysmopitheca Western apes Dentition 2. 1. 3. 3. |
26. Platyrrhinæ (Anthropops +) (Homunculus +) |
| Older Miocene | 27. Cynopitheca Dog-faced apes (tailed) | 27. Papiomorpha Cynocephalus |
| Neo-Miocene | 28. Anthropoides Man-like apes (tail-less) |
28. Hylobatida Hylobates Satyrus |
| Pliocene | 29. Pithecanthropi Ape-men (alali, speechless) | 29. Anthropitheca Chimpanzee Gorilla |
| Pleistocene | 30. Homines Men with speech | 30. Weddahs Australian negroes |
Our task of detecting the extinct ancestors of our race among the vast numbers of animals known to us encounters very different difficulties in the various sections of man’s stem-history. These were very great in the series of our invertebrate ancestors; they are much slighter in the subsequent series of our vertebrate ancestors. Within the vertebrate stem there is, as we have already seen, so complete an agreement in structure and embryology that it is impossible to doubt their phylogenetic unity. In this case the evidence is much clearer and more abundant.
The characteristics that distinguish the Vertebrates as a whole from the Invertebrates have already been discussed in our description of the hypothetical Primitive Vertebrate (Chapter XI, Figs. 98–102). The chief of these are: (1) The evolution of the primitive brain into a dorsal medullary tube; (2) the formation of the chorda between the medullary tube and the gut; (3) the division of the gut into branchial (gill) and hepatic (liver) gut; and (4) the internal articulation or metamerism. The first three features are shared by the Vertebrates with the ascidia-larvæ and the Prochordonia; the fourth is peculiar to them. Thus the chief advantage in organisation by which the earliest Vertebrates took precedence of the unsegmented Chordonia consisted in the development of internal segmentation.
The whole vertebrate stem divides first into the two chief sections of Acrania and Craniota. The Amphioxus is the only surviving representative of the older and lower section, the Acrania (“skull-less”). All the other vertebrates belong to the second division, the Craniota (“skull-animals”). The Craniota descend directly from the Acrania, and these from the primitive Chordonia. The exhaustive study that we made of the comparative anatomy and ontogeny of the Ascidia and the Amphioxus has proved these relations for us. (See Chapters XVI and XVII.) The Amphioxus, the lowest Vertebrate, and the Ascidia, the nearest related Invertebrate, descend from a common extinct stem-form, the Chordæa; and this must have had, substantially, the organisation of the chordula.
However, the Amphioxus is important not merely because it fills the deep gulf between the Invertebrates and Vertebrates, but also because it shows us to-day the typical vertebrate in all its simplicity. We owe to it the most important data that we proceed on in reconstructing the gradual historical development of the whole stem. All the Craniota descend from a common stem-form, and this was substantially identical in structure with the Amphioxus. This stem-form, the Primitive Vertebrate (Prospondylus, Figs. 98–102), had the characteristics of the vertebrate as such, but not the important features that distinguish the Craniota from the Acrania. Though the Amphioxus has many peculiarities of structure and has much degenerated, and though it cannot be regarded as an unchanged descendant of the Primitive Vertebrate, it must have inherited from it the specific characters we enumerated above. We may not say that “Amphioxus is the ancestor of the Vertebrates”; but we can say: “Amphioxus is the nearest relation to the ancestor of all the animals we know.” Both belong to the same small family, or lowest class of the Vertebrates, that we call the Acrania. In our genealogical tree this group forms the twelfth stage, or the first stage among the vertebrate ancestors (p. 228). From this group of Acrania both the Amphioxus and the Craniota were evolved.
The vast division of the Craniota embraces all the Vertebrates known to us, with the exception of the Amphioxus. All of them have a head clearly differentiated from the trunk, and a skull enclosing a brain. The head has also three pairs of higher sense-organs (nose, eyes, and ears). The brain is very rudimentary at first, a mere bulbous enlargement of the fore end of the medullary tube. But it is soon divided by a number of transverse constrictions into, first three, then five successive cerebral vesicles. In this formation of the head, skull, and brain, with further development of the higher sense-organs, we have the advance that the Craniota made beyond their skull-less ancestors. Other organs also attained a higher development; they acquired a compact centralised heart with valves and a more advanced liver and kidneys, and made progress in other important respects.
Fig. 247—The large marine lamprey (Petromyzon marinus), much reduced. Behind the eye there is a row of seven gill-clefts visible on the left, in front the round suctorial mouth.
We may divide the Craniota generally into Cyclostoma (“round-mouthed”) and Gnathostoma (“jaw-mouthed”). There are only a few groups of the former in existence now, but they are very interesting, because in their whole structure they stand midway between the Acrania and the Gnathostoma. They are much more advanced than the Acrania, much less so than the fishes, and thus form a very welcome connecting-link between the two groups. We may therefore consider them a special intermediate group, the fourteenth and fifteenth stages in the series of our ancestors.
The few surviving species of the Cyclostoma are divided into two orders—the Myxinoides and the Petromyzontes. The former, the hag-fishes, have a long, cylindrical, worm-like body. They were classed by Linné with the worms, and by later zoologists, with the fishes, or the amphibia, or the molluscs. They live in the sea, usually as parasites of fishes, into the skin of which they bore with their round suctorial mouths and their tongues, armed with horny teeth. They are sometimes found alive in the body cavity of fishes (such as the torsk or sturgeon); in these cases they have passed through the skin into the interior. The second order consists of the Petromyzontes or lampreys; the small river lamprey (Petromyzon fluviatilis) and the large marine lamprey (Petromyzon marinus, Fig. 247). They also have a round suctorial mouth, with horny teeth inside it; by means of this they attach themselves by sucking to fishes, stones, and other objects (hence the name Petromyzon = stone-sucker). It seems that this habit was very widespread among the earlier Vertebrates; the larvæ of many of the Ganoids and frogs have suctorial disks near the mouth.
The class that is formed of the Myxinoides and Petromyzontes is called the Cyclostoma (round-mouthed), because their mouth has a circular or semi-circular aperture. The jaws (upper and lower) that we find in all the higher Vertebrates are completely wanting in the Cyclostoma, as in the Amphioxus. Hence the other Vertebrates are collectively opposed to them as Gnathostoma (jaw-mouthed). The Cyclostoma might also be called Monorhina (single-nosed), because they have only a single nasal passage, while all the Gnathostoma have two nostrils (Amphirhina = double-nosed). But apart from these peculiarities the Cyclostoma differ more widely from the fishes in other special features of their structure than the fishes do from man. Hence they are obviously the last survivors of a very ancient class of Vertebrates, that was far from attaining the advanced organisation of the true fish. To mention only the chief points, the Cyclostoma show no trace of pairs of limbs. Their mucous skin is quite naked and smooth and devoid of scales. There is no bony skeleton. A very rudimentary skull is developed at the foremost end of their chorda. At this point a soft membranous (partly turning into cartilage), small skull-capsule is formed, and encloses the brain.
Fig. 248—Fossil Permian primitive fish (Pleuracanthus Dechenii), from the red sandstone of Saarbrücken. (From Döderlein.) I Skull and branchial skeleton: o eye-region, pq palatoquadratum, nd lower jaw, hm hyomandibular, hy tongue-bone, k gill-radii, kb gill-arches, z jaw-teeth, sz gullet-teeth, st neck-spine. II Vertebral column: ob upper arches, ub lower arches, hc intercentra, r ribs. III Single fins: d dorsal fin, c tail-fin (tail-end wanting), an anus-fin, ft supporter of fin-rays. IV Breast-fin: sg shoulder-zone, ax fin-axis, ss double lines of fin-rays, bs additional rays, sch plates. V Ventral fin: p pelvis, ax fin-axis, ss single row of fin-rays, bs additional rays, sch scales, cop penis.
The brain of the Cyclostoma is merely a very small and comparatively insignificant swelling of the spinal marrow, a simple vesicle at first. It afterwards divides into five successive cerebral vesicles, like the brain of the Gnathostoma. These five primitive cerebral vesicles, that are found in the embryos of all the higher vertebrates from the fishes to man, and grow into very complex structures, remain at a very rudimentary stage in the Cyclostoma. The histological structure of the nerves is also less advanced than in the rest of the vertebrates. In these the auscultory organ always contains three circular canals, but in the lampreys there are only two, and in the hag-fishes only one. In most other respects the organisation of the Cyclostoma is much simpler—for instance, in the structure of the heart, circulation, and kidneys. We must especially note the absence of a very important organ that we find in the fishes, the floating-bladder, from which the lungs of the higher Vertebrates have been developed.
When we consider all these peculiarities in the structure of the Cyclostoma, we may formulate the following thesis: Two divergent lines proceeded from the earliest Craniota, or the primitive Craniota (Archicrania). One of these lines is preserved in a greatly modified condition: these are the Cyclostoma, a very backward and partly degenerate side-line. The other, the chief line of the Vertebrate stem, advanced straight to the fishes, and by fresh adaptations acquired a number of important improvements.
The Cyclostoma are almost always classified by zoologists among the fishes; but the incorrectness of this may be judged from the fact that in all the chief and distinctive features of organisation they are further removed from the fishes than the fishes are from the Mammals, and even man. With the fishes we enter upon the vast division of the jaw-mouthed or double-nosed Vertebrates (Gnathostoma or Amphirhina). We have to consider the fishes carefully as the class which, on the evidence of palæontology, comparative anatomy, and ontogeny, may be regarded with absolute certainty as the stem-class of all the higher Vertebrates or Gnathostomes. Naturally, none of the actual fishes can be considered the direct ancestor of the higher Vertebrates. But it is certain that all the Vertebrates or Gnathostomes, from the fishes to man, descend from a common, extinct, fish-like ancestor. If we had this ancient stem-form before us, we would undoubtedly class it as a true fish. Fortunately the comparative anatomy and classification of the fishes are now so far advanced that we can get a very clear idea of these interesting and instructive features.
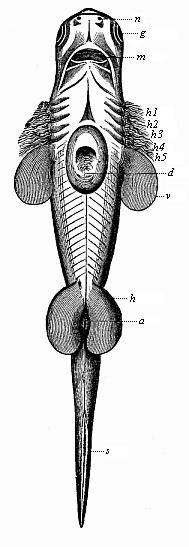
Fig. 249—Embryo of a shark (Scymnus lichia), seen from the ventral side. v breast-fins (in front five pairs of gill-clefts), h belly-fins, a anus, s tail-fin, k external gill-tuft, d yelk-sac (removed for most part), g eye, n nose, m mouth-cleft.
In order to understand properly the genealogical tree of our race within the vertebrate stem, it is important to bear in mind the characteristics that separate the whole of the Gnathostomes from the Cyclostomes and Craniota. In these respects the fishes agree entirely with all the other Gnathostomes up to man, and it is on this that we base our claim of relationship to the fishes. The following characteristics of the Gnathostomes are anatomic features of this kind: (1) The internal gill-arch apparatus with the jaw arches; (2) the pair of nostrils; (3) the floating bladder or lungs; and (4) the two pairs of limbs.
The peculiar formation of the frame work of the branchial (gill) arches and the connected maxillary (jaw) apparatus is of importance in the whole group of the Gnathostomes. It is inherited in rudimentary form by all of them, from the earliest fishes to man. It is true that the primitive transformation (which we find even in the Ascidia) of the fore gut into the branchial gut can be traced in all the Vertebrates to the same simple type; in this respect the gill-clefts, which pierce the walls of the branchial gut in all the Vertebrates and in the Ascidia, are very characteristic. But the external, superficial branchial skeleton that supports the gill-crate in the Cyclostoma is replaced in the Gnathostomes by an internal branchial skeleton. It consists of a number of successive cartilaginous arches, which lie in the wall of the gullet between the gill-clefts, and run round the gullet from both sides. The foremost pair of gill-arches become the maxillary arches, from which we get our upper and lower jaws.
The olfactory organs are at first found in the same form in all the Gnathostomes, as a pair of depressions in the fore part of the skin of the head, above the mouth; hence, they are also called the Amphirhina (“double-nosed”). The Cyclostoma are “one-nosed” (Monorhina); their nose is a single passage in the middle of the frontal surface. But as the olfactory nerve is double in both cases, it is possible that the peculiar form of the nose in the actual Cyclostomes is a secondary acquisition (by adaptation to suctorial habits).
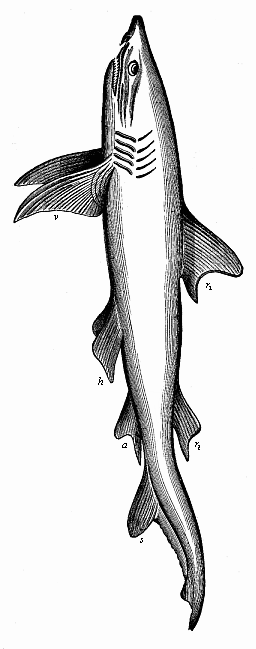
Fig. 250—Fully developed man-eating shark (Carcharias melanopterus), left view. r1 first, r2 second dorsal fin, s tail-fin, a anus-fin, v breast-fins, h belly-fins.)
A third essential character of the Gnathostomes, that distinguishes them very conspicuously from the lower vertebrates we have dealt with, is the formation of a blind sac by invagination from the fore part of the gut, which becomes in the fishes the air-filled floating-bladder. This organ acts as a hydrostatic apparatus, increasing or reducing the specific gravity of the fish by compressing or altering the quantity of air in it. The fish can rise or sink in the water by means of it. This is the organ from which the lungs of the higher vertebrates are developed.
Finally, the fourth character of the Gnathostomes in their simple embryonic form is the two pairs of extremities or limbs—a pair of fore legs (breast-fins in the fish, Fig. 250 v) and a pair of hind legs (ventral fins in the fish, Fig. 250 h). The comparative anatomy of these fins is very interesting, because they contain the rudiments of all the skeletal parts that form the framework of the fore and hind legs in all the higher vertebrates right up to man. There is no trace of these pairs of limbs in the Acrania and Cyclostomes.
Turning, now, to a closer inspection of the fish class, we may first divide it into three groups or sub-classes, the genealogy of which is well known to us. The first and oldest group is the sub-class of the Selachii or primitive fishes; the best-known representatives of which to-day are the orders of the sharks and rays (Figs. 248–252). Next to this is the more advanced sub-class of the plated fishes or Ganoids (Figs. 253–5). It has been long extinct for the most part, and has very few living representatives, such as the sturgeon and the bony pike; but we can form some idea of the earlier extent of this interesting group from the large numbers of fossils. From these plated fishes the sub-class of the bony fishes or Teleostei was developed, to which the great majority of living fishes belong (especially nearly all our river fishes). Comparative anatomy and ontogeny show clearly that the Ganoids descended from the Selachii, and the Teleostei from the Ganoids. On the other hand, a collateral line, or rather the advancing chief line of the vertebrate stem, was developed from the earlier Ganoids, and this leads us through the group of the Dipneusta to the important division of the Amphibia.
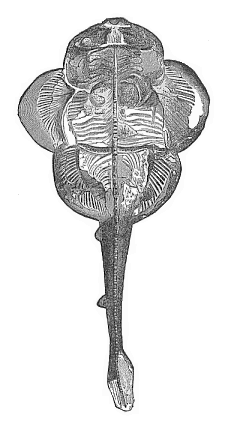
Fig. 251—Fossil angel-shark (Squatina alifera), from the upper Jurassic at Eichstätt. (From Zittel.) The cartilaginous skull is clearly seen in the broad head, and the gill-arches behind. The wide breast-fin and the narrower belly-fin have a number of radii; between these and the vertebral column are a number of ribs.
The earliest fossil remains of Vertebrates that we know were found in the Upper Silurian (p. 201), and belong to two groups—the Selachii and the Ganoids. The most primitive of all known representatives of the earliest fishes are probably the remarkable Pleuracanthida, the genera Pleuracanthus, Xenacanthus, Orthocanthus, etc. (Fig. 248). These ancient cartilaginous fishes agree in most points of structure with the real sharks (Figs. 249, 250); but in other respects they seem to be so much simpler in organisation that many palæontologists separate them altogether, and regard them as Proselachii; they are probably closely related to the extinct ancestors of the Gnathostomes. We find well-preserved remains of them in the Permian period. Well-preserved impressions of other sharks are found in the Jurassic schist, such as of the angel-fish (Squatina, Fig. 251). Among the extinct earlier sharks of the Tertiary period there were some twice as large as the biggest living fishes; Carcharodon was more than 100 feet long. The sole surviving species of this genus (C. Rondeleti) is eleven yards long, and has teeth two inches long; but among the fossil species we find teeth six inches long (Fig. 252).
From the primitive fishes or Selachii, the earliest Gnathostomes, was developed the legion of the Ganoids. There are very few genera now of this interesting and varied group—the ancient sturgeons (Accipenser), the eggs of which are eaten as caviare, and the stratified pikes (Polypterus, Fig. 255) in African rivers, and bony pikes (Lepidosteus) in the rivers of North America. On the other hand, we have a great variety of specimens of this group in the fossil state, from the Upper Silurian onward. Some of these fossil Ganoids approach closely to the Selachii; others are nearer to the Dipneusts; others again represent a transition to the Teleostei. For our genealogical purposes the most interesting are the intermediate forms between the Selachii and the Dipneusts. Huxley, to whom we owe particularly important works on the fossil Ganoids, classed them in the order of the Crossopterygii. Many genera and species of this order are found in the Devonian and Carboniferous strata (Fig. 253); a single, greatly modified survivor of the group is still found in the large rivers of Africa (Polypterus, Fig. 255, and the closely related Calamichthys). In many impressions of the Crossopterygii the floating bladder seems to be ossified, and therefore well preserved—for instance, in the Undina (Fig. 254, immediately behind the head).
Part of these Crossopterygii approach very closely in their chief anatomic features to the Dipneusts, and thus represent phylogenetically the transition from the Devonian Ganoids to the earliest air-breathing vertebrates. This important advance was made in the Devonian period. The numerous fossils that we have from the first two geological sections, the Laurentian and Cambrian periods, belong exclusively to aquatic plants and animals. From this paleontological fact, in conjunction with important geological and biological indications, we may infer with some confidence that there were no terrestrial animals at that time. During the whole of the vast archeozoic period—many millions of years—the living population of our planet consisted almost exclusively of aquatic organisms; this is a very remarkable fact, when we remember that this period embraces the larger half of the whole history of life. The lower animal-stems are wholly (or with very few exceptions) aquatic. But the higher stems also remained in the water during the primordial epoch. It was only towards its close that some of them came to live on land. We find isolated fossil remains of terrestrial animals first in the Upper Silurian, and in larger numbers in the Devonian strata, which were deposited at the beginning of the second chief section of geology (the paleozoic age). The number increases considerably in the Carboniferous and Permian deposits. We find many species both of the articulate and the vertebrate stem that lived on land and breathed the atmosphere; their aquatic ancestors of the Silurian period only breathed water. This important change in respiration is the chief modification that the animal organism underwent in passing from the water to the solid land. The first consequence was the formation of lungs for breathing air; up to that time the gills alone had served for respiration. But there was at the same time a great change in the circulation and its organs; these are always very closely correlated to the respiratory organs. Moreover, the limbs and other organs were also more or less modified, either in consequence of remote correlation to the preceding or owing to new adaptations.
Fig. 252—Tooth of a gigantic shark (Carcharodon megalodon), from the Pliocene at Malta. (From Zittel.)
In the vertebrate stem it was unquestionably a branch of the fishes—in fact, of the Ganoids—that made the first fortunate experiment during the Devonian period of adapting themselves to terrestrial life and breathing the atmosphere. This led to a modification of the heart and the nose. The true fishes have merely a pair of blind olfactory pits on the surface of the head; but a connection of these with the cavity of the mouth was now formed. A canal made its appearance on each side, and led directly from the nasal depression into the mouth-cavity, thus conveying atmospheric air to the lungs even when the mouth was closed. Further, in all true fishes the heart has only two sections—an atrium that receives the venous blood from the veins, and a ventricle that propels it through a conical artery to the gills; the atrium was now divided into two halves, or right and left auricles, by an incomplete partition. The right auricle alone now received the venous blood from the body, while the left auricle received the venous blood that flowed from the lungs and gills to the heart. Thus the double circulation of the higher vertebrates was evolved from the simple circulation of the true fishes, and, in accordance with the laws of correlation, this advance led to others in the structure of other organs.
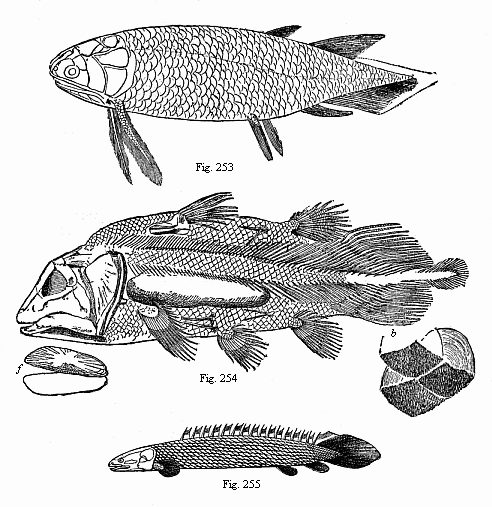
Fig. 253—A Devonian Crossopterygius
(Holoptychius nobilissimus), from the Scotch old red sandstone. (From
Huxley.)
Fig. 254.—A Jurassic Crossopterygius
(Undina penicillata), from the upper Jurassic at Eichstätt. (From
Zittel.) j jugular plates, b three ribbed scales.
Fig. 255—A living Crossopterygius, from the Upper Nile
((Polypterus bichir).
The vertebrate class, that thus adapted itself to breathing the atmosphere, and was developed from a branch of the Ganoids, takes the name of the Dipneusts or Dipnoa (“double-breathers”), because they retained the earlier gill-respiration along with the new pulmonary (lung) respiration, like the lowest amphibia. This class was represented during the paleozoic age (or the Devonian, Carboniferous, and Permian periods) by a number of different genera. There are only three genera of the class living to-day: Protopterus annectens in the rivers of tropical Africa (the White Nile, the Niger, Quelliman, etc.), Lepidosiren paradoxa in tropical South America (in the tributaries of the Amazon), and Ceratodus Forsteri in the rivers of East Australia. This wide distribution of the three isolated survivors proves that they represent a group that was formerly very large. In their whole structure they form a transition from the fishes to the amphibia. The transitional formation between the two classes is so pronounced in the whole organisation of these remarkable animals that zoologists had a lively controversy over the question whether they were really fishes or amphibia. Several distinguished zoologists classed them with the amphibia, though most now associate them with the fishes. As a matter of fact, the characters of the two classes are so far united in the Dipneusts that the answer to the question depends entirely on the definition we give of “fish” and “amphibian.” In habits they are true amphibia. During the tropical winter, in the rainy season, they swim in the water like the fishes, and breathe water by gills. During the dry season they bury themselves in the dry mud, and breathe the atmosphere through lungs, like the amphibia and the higher vertebrates. In this double respiration they resemble the lower amphibia, and have the same characteristic formation of the heart; in this they are much superior to the fishes. But in most other features they approach nearer to the fishes, and are inferior to the amphibia. Externally they are entirely fish-like.
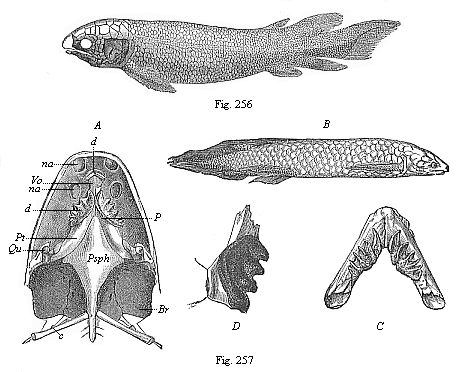
Fig. 256—Fossil Dipneust (Dipterus
Valenciennesi), from the old red sandstone (Devon). (From
Pander.)
Fig. 257—The Australian Dipneust
(Ceratodus Forsteri). B view from the right, A lower side
of the skull, C lower jaw. (From Gunther.) Qu quadrate
bone, Psph parasphenoid, Pt P pterygopalatinum, Vo vomer,
d teeth, na nostrils, Br branchial cavity, C first
rib. D lower-jaw teeth of the fossil Ceratodus Kaupi (from the
Triassic).
In the Dipneusts the head is not marked off from the trunk. The skin is covered with large scales. The skeleton is soft, cartilaginous, and at a low stage of development, as in the lower Selachii and the earliest Ganoids. The chorda is completely retained, and surrounded by an unsegmented sheath. The two pairs of limbs are very simple fins of a primitive type, like those of the lowest Selachii. The formation of the brain, the gut, and the sexual organs is also the same as in the Selachii. Thus the Dipneusts have preserved by heredity many of the less advanced features of our primitive fish-like ancestors, and at the same time have made a great step forward in adaptation to air-breathing by means of lungs and the correlative improvement of the heart.
Fig. 258—Young ceratodus, shortly after
issuing from the egg, magnified. k gill-cover,
l liver. (From
Richard Semon.)
Fig. 259—Young ceratodus six weeks
after issuing from the egg. s spiral fold of gut,
b
rudimentary belly-fin. (From Richard Semon.)
Ceratodus is particularly interesting on account of the primitive build of its skeleton; the cartilaginous skeleton of its two pairs of fins, for instance, has still the original form of a bi-serial or feathered leaf, and was on that account described by Gegenbaur as a “primitive fin-skeleton.” On the other hand, the skeleton of the pairs of fins is greatly reduced in the African dipneust (Protopterus) and the American (Lepidosiren). Further, the lungs are double in these modern dipneusts, as in all the other air-breathing vertebrates; they have on that account been called “double-lunged” (Dipneumones) in contrast to the Ceratodus; the latter has only a single lung (Monopneumones). At the same time the gills also are developed as water-breathing organs in all these lung-fishes. Protopterus has external as well as internal gills.
The paleozoic Dipneusts that are in the direct line of our ancestry, and form the connecting-bridge between the Ganoids and the Amphibia, differ in many respects from their living descendants, but agree with them in the above essential features. This is confirmed by a number of interesting facts that have lately come to our knowledge in connection with the embryonic development of the Ceratodus and Lepidosiren; they give us important information as to the stem-history of the lower Vertebrates, and therefore of our early ancestors of the paleozoic age.
With the phylogenetic study of the four higher classes of Vertebrates, which must now engage our attention, we reach much firmer ground and more light in the construction of our genealogy than we have, perhaps, enjoyed up to the present. In the first place, we owe a number of very valuable data to the very interesting class of Vertebrates that come next to the Dipneusts and have been developed from them—the Amphibia. To this group belong the salamander, the frog, and the toad. In earlier days all the reptiles were, on the example of Linne, classed with the Amphibia (lizards, serpents, crocodiles, and tortoises). But the reptiles are much more advanced than the Amphibia, and are nearer to the birds in the chief points of their structure. The true Amphibia are nearer to the Dipneusta and the fishes; they are also much older than the reptiles. There were plenty of highly-developed (and sometimes large) Amphibia during the Carboniferous period; but the earliest reptiles are only found in the Permian period. It is probable that the Amphibia were evolved even earlier—during the Devonian period—from the Dipneusta. The extinct Amphibia of which we have fossil remains from that remote period (very numerous especially in the Triassic strata) were distinguished for a graceful scaly coat or a powerful bony armour on the skin (like the crocodile), whereas the living amphibia have usually a smooth and slippery skin.
The earliest of these armoured Amphibia (Phractamphibia) form the order of Stegocephala (“roof-headed”) (Fig. 260). It is among these, and not among the actual Amphibia, that we must look for the forms that are directly related to the genealogy of our race, and are the ancestors of the three higher classes of Vertebrates. But even the existing Amphibia have such important relations to us in their anatomic structure, and especially their embryonic development, that we may say: Between the Dipneusts and the Amniotes there was a series of extinct intermediate forms which we should certainly class with the Amphibia if we had them before us. In their whole organisation even the actual Amphibia seem to be an instructive transitional group. In the important respects of respiration and circulation they approach very closely to the Dipneusta, though in other respects they are far superior to them.
This is particularly true of the development of their limbs or extremities. In them we find these for the first time as five-toed feet. The thorough investigations of Gegenbaur have shown that the fish’s fins, of which very erroneous opinions were formerly held, are many-toed feet. The various cartilaginous or bony radii that are found in large numbers in each fin correspond to the fingers or toes of the higher Vertebrates. The several joints of each fin-radius correspond to the various parts of the toe. Even in the Dipneusta the fin is of the same construction as in the fishes; it was afterwards gradually evolved into the five-toed form, which we first encounter in the Amphibia. This reduction of the number of the toes to six, and then to five, probably took place in the second half of the Devonian period—at the latest, in the subsequent Carboniferous period—in those Dipneusta which we regard as the ancestors of the Amphibia. We have several fossil remains of five-toed Amphibia from this period. There are numbers of fossil impressions of them in the Triassic of Thuringia (Chirotherium).
The fact that the toes number five is of great importance, because they have clearly been transmitted from the Amphibia to all the higher Vertebrates. Man entirely resembles his amphibian ancestors in this respect, and indeed in the whole structure of the bony skeleton of his five-toed extremities. A careful comparison of the skeleton of the frog with our own is enough to show this. It is well known that this hereditary number of the toes has assumed a very great practical importance from remote times; on it our whole system of enumeration (the decimal system applied to measurement of time, mass, weight, etc.) is based. There is absolutely no reason why there should be five toes in the fore and hind feet in the lowest Amphibia, the reptiles, and the higher Vertebrates, unless we ascribe it to inheritance from a common stem-form. Heredity alone can explain it. It is true that we find less than five toes in many of the Amphibia and of the higher Vertebrates. But in all these cases we can prove that some of the toes atrophied, and were in time lost altogether.
Fig. 260—Fossil amphibian from the Permian, found in the Plauen terrain near Dresden (Branchiosaurus amblystomus). (From Credner.) A skeleton of a young larva. B larva, restored, with gills. C the adult form.)
The causes of this evolution of the five-toed foot from the many-toed fin in the amphibian ancestor must be sought in adaptation to the entire change of function that the limbs experienced in passing from an exclusively aquatic to a partly terrestrial life. The many-toed fin had been used almost solely for motion in the water; it had now also to support the body in creeping on the solid ground. This led to a modification both of the skeleton and the muscles of the limbs. The number of the fin-radii was gradually reduced, and sank finally to five. But these five remaining radii became much stronger. The soft cartilaginous radii became bony rods. The rest of the skeleton was similarly strengthened. Thus from the one-armed lever of the many-toed fish-fin arose the improved many-armed lever system of the five-toed amphibian limbs. The movements of the body gained in variety as well as in strength. The various parts of the skeletal system and correlated muscular system began to differentiate more and more. In view of the close correlation of the muscular and nervous systems, this also made great advance in structure and function. Hence we find, as a matter of fact, that the brain is much more developed in the higher Amphibia than in the fishes, the Dipneusta, and the lower Amphibia.
Fig. 261—Larva of the Spotted Salamander (Salamandra maculata), seen from the ventral side. In the centre a yelk-sac still hangs from the gut. The external gills are gracefully ramified. The two pairs of legs are still very small.
The first advance in organisation that was occasioned by the adoption of life on land was naturally the construction of an organ for breathing air—a lung. This was formed directly from the floating-bladder inherited from the fishes. At first its function was insignificant beside that of the gills, the older organ for water-respiration. Hence we find in the lowest Amphibia, the gilled Amphibia, that, like the Dipneusta, they pass the greater part of their life in the water, and breathe water through gills. They only come to the surface at brief intervals, or creep on to the land, and then breathe air by their lungs. But some of the tailed Amphibia—the salamanders—remain entirely in the water when they are young, and afterwards spend most of their time on land. In the adult state they only breathe air through lungs. The same applies to the most advanced of the Amphibia, the Batrachia (frogs and toads); some of them have entirely lost the gill-bearing larva form.[30] This is also the case with certain small, serpentine Amphibia, the Cæcilia (which live in the ground like earth-worms).
[30] The tree-frog of Martinique (Hylades martinicensis) loses the gills on the seventh, and the tail and yelk-sac on the eighth, day of fœtal life. On the ninth or tenth day after fecundation the frog emerges from the egg.
The great interest of the natural history of the Amphibia consists especially in their intermediate position between the lower and higher Vertebrates. The lower Amphibia approach very closely to the Dipneusta in their whole organisation, live mainly in the water, and breathe by gills; but the higher Amphibia are just as close to the Amniotes, live mainly on land, and breathe by lungs. But in their younger state the latter resemble the former, and only reach the higher stage by a complete metamorphosis. The embryonic development of most of the higher Amphibia still faithfully reproduces the stem-history of the whole class, and the various stages of the advance that was made by the lower Vertebrates in passing from aquatic to terrestrial life during the Devonian or the Carboniferous period are repeated in the spring by every frog that develops from an egg in our ponds.
Fig. 262—Larva of the common grass-frog (Rana temporaria), or “tadpole.” m mouth, n a pair of suckers for fastening on to stones, d skin-fold from which the gill-cover develops; behind it the gill-clefts, from which the branching gills (k) protrude, s tail-muscles, f cutaneous fin-fringe of the tail.
The common frog leaves the egg in the shape of a larva, like the tailed salamander (Fig. 261), and this is altogether different from the mature frog (Fig. 262). The short trunk ends in a long tail, with the form and structure of a fish’s tail (s). There are no limbs at first. The respiration is exclusively branchial, first through external (k) and then internal gills. In harmony with this the heart has the same structure as in the fish, and consists of two sections—an atrium that receives the venous blood from the body, and a ventricle that forces it through the arteries into the gills.
We find the larvæ of the frog (or tadpoles, Gyrini) in great numbers in our ponds every spring in this fish-form, using their muscular tails in swimming, just like the fishes and young Ascidia. When they have reached a certain size, the remarkable metamorphosis from the fish-form to the frog begins. A blind sac grows out of the gullet, and expands into a couple of spacious sacs: these are the lungs. The simple chamber of the heart is divided into two sections by the development of a partition, and there are at the same time considerable changes in the structure of the chief arteries. Previously all the blood went from the auricle through the aortic arches into the gills, but now only part of it goes to the gills, the other part passing to the lungs through the new-formed pulmonary artery. From this point arterial blood returns to the left auricle of the heart, while the venous blood gathers in the right auricle. As both auricles open into a single ventricle, this contains mixed blood. The dipneust form has now succeeded to the fish-form. In the further course of the metamorphosis the gills and the branchial vessels entirely disappear, and the respiration becomes exclusively pulmonary. Later, the long swimming tail is lost, and the frog now hops to the land with the legs that have grown meantime.
This remarkable metamorphosis of the Amphibia is very instructive in connection with our human genealogy, and is particularly interesting from the fact that the various groups of actual Amphibia have remained at different stages of their stem-history, in harmony with the biogenetic law. We have first of all a very low order of Amphibia—the Sozobranchia (“gilled-amphibia”), which retain their gills throughout life, like the fishes. In a second order of the salamanders the gills are lost in the metamorphosis, and when fully grown they have only pulmonary respiration. Some of the tailed Amphibia still retain the gill-clefts in the side of the neck, though they have lost the gills themselves (Menopoma). If we force the larvæ of our salamanders (Fig. 261) and tritons to remain in the water, and prevent them from reaching the land, we can in favourable circumstances make them retain their gills. In this fish-like condition they reach sexual maturity, and remain throughout life at the lower stage of the gilled Amphibia.
fish-like axolotl (Siredon pisciformis). It was formerly regarded as a permanent gilled amphibian persisting throughout life at the fish-stage. But some of the hundreds of these animals that are kept in the Botanical Garden at Paris got on to the land for some reason or other, lost their gills, and changed into a form closely resembling the salamander (Amblystoma). Other species of the genus became sexually mature for the first time in this condition. This has been regarded as an astounding phenomenon, although every common frog and salamander repeats the metamorphosis in the spring. The whole change from the aquatic and gill-breathing animal to the terrestrial lung-breathing form may be followed step by step in this case. But what we see here in the development of the individual has happened to the whole class in the course of its stem-history.
Fig. 263—Fossil mailed amphibian, from the Bohemian Carboniferous (Seeleya). (From Fritsch.) The scaly coat is retained on the left.
The metamorphosis goes farther in a third order of Amphibia, the Batrachia or Anura, than in the salamander. To this belong the various kinds of toads, ringed snakes, water-frogs, tree-frogs, etc. These lose, not only the gills, but also (sooner or later) the tail, during metamorphosis.
The ontogenetic loss of the gills and the tail in the frog and toad can only be explained on the assumption that they are descended from long-tailed Amphibia of the salamander type. This is also clear from the comparative anatomy of the two groups. This remarkable metamorphosis is, however, also interesting because it throws a certain light on the phylogeny of the tail-less apes and man. Their ancestors also had long tails and gills like the gilled Amphibia, as the tail and the gill-arches of the human embryo clearly show.
For comparative anatomical and ontogenetic reasons, we must not seek these amphibian ancestors of ours—as one would be inclined to do, perhaps—among the tail-less Batrachia, but among the tailed lower Amphibia.
The vertebrate form that comes next to the Amphibia in the series of our ancestors is a lizard-like animal, the earlier existence of which can be confidently deduced from the facts of comparative anatomy and ontogeny. The living Hatteria of New Zealand (Fig. 264) and the extinct Rhyncocephala of the Permian period (Fig. 265) are closely related to this important stem-form; we may call them the Protamniotes, or Primitive Amniotes. All the Vertebrates above the Amphibia—or the three classes of reptiles, birds, and mammals—differ so much in their whole organisation from all the lower Vertebrates we have yet considered, and have so great a resemblance to each other, that we put them all together in a single group with the title of Amniotes. In these three classes alone we find the remarkable embryonic membrane, already mentioned, which we called the amnion; a cenogenetic adaptation that we may regard as a result of the sinking of the growing embryo into the yelk-sac.
All the Amniotes known to us—all reptiles, birds, and mammals (including man)—agree in so many important points of internal structure and development that their descent from a common ancestor can be affirmed with tolerable certainty. If the evidence of comparative anatomy and ontogeny is ever entirely beyond suspicion, it is certainly the case here. All the peculiarities that accompany and follow the formation of the amnion, and that we have learned in our consideration of human embryology; all the peculiarities in the development of the organs which we will presently follow in detail; finally, all the principal special features of the internal structure of the full-grown Amniotes—prove so clearly the common origin of all the Amniotes from single extinct stem-form that it is difficult to entertain the idea of their evolution from several independent stems. This unknown common stem-form is our primitive Amniote (Protamnion). In outward appearance it was probably something between the salamander and the lizard.
It is very probable that some part of the Permian period was the age of the origin of the Protamniotes. This follows from the fact that the Amphibia are not fully developed until the Carboniferous period, and that the first fossil reptiles (Palæhatteria, Homœosaurus, Proterosaurus) are found towards the close of the Permian period. Among the important changes of the vertebrate organisation that marked the rise of the first Amniotes from salamandrine Amphibia during this period the following three are especially noteworthy: the entire disappearance of the water-breathing gills and the conversion of the gill-arches into other organs, the formation of the allantois or primitive urinary sac, and the development of the amnion.
One of the most salient characteristics of the Amniotes is the complete loss of the gills. All Amniotes, even if living in water (such as sea-serpents and whales), breathe air through lungs, never water through gills. All the Amphibia (with very rare exceptions) retain their gills for some time when young, and have for a time (if not permanently) branchial respiration; but after these there is no question of branchial respiration. The Protamniote itself must have entirely abandoned water-breathing. Nevertheless, the gill-arches are preserved by heredity, and develop into totally different (in part rudimentary) organs—various parts of the bone of the tongue, the frame of the jaws, the organ of hearing, etc. But we do not find in the embryos of the Amniotes any trace of gill-leaves, or of real respiratory organs on the gill-arches.
With this complete abandonment of the gills is probably connected the formation of another organ, to which we have already referred in embryology—namely, the allantois or primitive urinary sac (cf. p. 166). It is very probable that the urinary bladder of the Dipneusts is the first structure of the allantois. We find in these a urinary bladder that proceeds from the lower wall of the hind end of the gut, and serves as receptacle for the renal secretions. This organ has been transmitted to the Amphibia, as we can see in the frog.
The formation of the amnion and the allantois and the complete disappearance of the gills are the chief characteristics that distinguish the Amniotes from the lower Vertebrates we have hitherto considered. To these we may add several subordinate features that are transmitted to all the Amniotes, and are found in these only. One striking embryonic character of the Amniotes is the great curve of the head and neck in the embryo. We also find an advance in the structure of several of the internal organs of the Amniotes which raises them above the highest of the anamnia. In particular, a partition is formed in the simple ventricle of the heart, dividing into right and left chambers. In connection with the complete metamorphosis of the gill-arches we find a further development of the auscultory organs. Also, there is a great advance in the structure of the brain, skeleton, muscular system, and other parts. Finally, one of the most important changes is the reconstruction of the kidneys. In all the earlier Vertebrates we have found the primitive kidneys as excretory organs, and these appear at an early stage in the embryos of all the higher Vertebrates up to man. But in the Amniotes these primitive kidneys cease to act at an early stage of embryonic life, and their function is taken up by the permanent or secondary kidneys, which develop from the terminal section of the prorenal ducts.
Taking all these peculiarities of the Amniotes together, it is impossible to doubt that all the animals of this group—all reptiles, birds, and mammals—have a common origin, and form a single blood-related stem. Our own race belongs to this stem. Man is, in every feature of his organisation and embryonic development, a true Amniote, and has descended from the Protamniote with all the other Amniotes. Though they appeared at the end (possibly even in the middle) of the Paleozoic age, the Amniotes only reached their full development during the Mesozoic age. The birds and mammals made their first appearance during this period. Even the reptiles show their greatest growth at this time, so that it is called “the reptile age.” The extinct Protamniote, the ancestor of the whole group, belongs in its whole organisation to the reptile class.
Fig. 264—The lizard (Hatteria punctata = Sphenodon punctatus) of New Zealand. The sole surviving proreptile. (From Brehm.)
The genealogical tree of the amniote group is clearly indicated in its chief lines by their paleontology, comparative anatomy, and ontogeny. The group succeeding the Protamniote divided into two branches. The branch that will claim our whole interest is the class of the Mammals. The other branch, which developed in a totally different direction, and only comes in contact with the Mammals at its root, is the combined group of the reptiles and birds; these two classes may, with Huxley, be conveniently grouped together as the Sauropsida. Their common stem-form is an extinct lizard-like reptile of the order of the Rhyncocephalia. From this have been developed in various directions the serpents, crocodiles, tortoises, etc.—in a word, all the members of the reptile class. But the remarkable class of the birds has also been evolved directly from a branch of the reptile group, as is now established beyond question. The embryos of the reptiles and birds are identical until a very late stage, and have an astonishing resemblance even later. Their whole structure agrees so much that no anatomist now questions the descent of the birds from the reptiles. On the other hand, the mammal line has descended from the group of the Sauromammalia, a different branch of the Proreptilia. It is connected at its deepest roots with the reptile line, but it then diverges completely from it and follows a distinctive development. Man is the highest outcome of this class, the “crown of creation.” The hypothesis that the three higher Vertebrate classes represent a single Amniote-stem, and that the common root of this stem is to be found in the amphibian class, is now generally admitted.
The instructive group of the Permian Tocosauria, the common root from which the divergent stems of the Sauropsids and mammals have issued, merits our particular attention as the stem-group of all the Amniotes. Fortunately a living representative of this extinct ancestral group has been preserved to our day; this is the remarkable lizard of New Zealand, Hatteria punctata (Fig. 264). Externally it differs little from the ordinary lizard; but in many important points of internal structure, especially in the primitive construction of the vertebral column, the skull, and the limbs, it occupies a much lower position, and approaches its amphibian ancestors, the Stegocephala. Hence Hatteria is the phylogenetically oldest of all living reptiles, an isolated survivor from the Permian period, closely resembling the common ancestor of the Amniotes. It must differ so little from this extinct form, our hypothetical Protamniote, that we put it next to the Proreptilia. The remarkable Permian Palæhatteria, that Credner discovered in the Plauen terrain at Dresden in 1888, belongs to the same group (Fig. 266). The Jurassic genus Homœosaurus (Fig. 265), of which well-preserved skeletons are found in the Solenhofen schists, is perhaps still more closely related to them.
Unfortunately, the numerous fossil remains of Permian and Triassic Tocosauria that we have found in the last two decades are, for the most part, very imperfectly preserved. Very often we can make only precarious inferences from these skeletal fragments as to the anatomic characters of the soft parts that went with the bony skeleton of the extinct Tocosauria. Hence it has not yet been possible to arrange these important fossils with any confidence in the ancestral series that descend from the Protamniotes to the Sauropsids on the one side and the Mammals on the other. Opinions are particularly divided as to the place in classification and the phylogenetic significance of the remarkable Theromorpha. Cope gives this name to a very interesting and extensive group of extinct terrestrial reptiles, of which we have only fossil remains from the Permian and Triassic strata. Forty years ago some of these Therosauria (fresh-water animals) were described by Owen as Anomodontia. But during the last twenty years the distinguished American paleontologists, Cope and Osborn, have greatly increased our knowledge of them, and have claimed that the stem-forms of the Mammals must be sought in this order. As a matter of fact, the Theromorpha are nearer to the Mammals in the chief points of structure than any other reptiles. This is especially true of the Thereodontia, to which the Pureosauria and Pelycosauria belong (Fig. 267). The whole structure of their pelvis and hind-feet has attained the same form as in the Monotremes, the lowest Mammals. The formation of the scapula and the quadrate bone shows an approach to the Mammals such as we find in no other group of reptiles. The teeth also are already divided into incisors, canines, and molars. Nevertheless, it is very doubtful whether the Theromorpha really are in the ancestral line of the Sauromammals, or lead direct from the Tocosauria to the earliest Mammals. Other experts on this group believe that it is an independent legion of the reptiles, connected, perhaps, at its lowest root, with the Sauromammals, but developed quite independently of the Mammals—though parallel to them in many ways.
One of the most important of the zoological facts that we rely on in our investigation of the genealogy of the human race is the position of man in the Mammal class. However different the views of zoologists may have been as to this position in detail, and as to his relations to the apes, no scientist has ever doubted that man is a true mammal in his whole organisation and development. Linné drew attention to this fact in the first edition of his famous Systema Naturæ (1735). As will be seen in any museum of anatomy or any manual of comparative anatomy; the human frame has all the characteristics that are common to the Mammals and distinguish them conspicuously from all other animals.
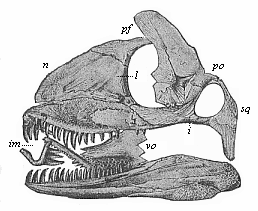
Fig. 266—Skull of a Permian lizard (Palæhatteria longicaudata). (From Credner.) n nasal bone, pf frontal bone, l lachrymal bone, po postorbital bone, sq covering bone, i cheek-bone, vo vomer, im inter-maxillary.
If we examine this undoubted fact from the point of view of phylogeny, in the light of the theory of descent, it follows at once that man is of a common stem with all the other Mammals, and comes from the same root as they. But the various features in which the Mammals agree and by which they are distinguished are of such a character as to make a polyphyletic hypothesis quite inadmissible. It is impossible to entertain the idea that all the living and extinct Mammals come from a number of separate roots. If we accept the general theory of evolution, we are bound to admit the monophyletic hypothesis of the descent of all the Mammals (including man) from a single mammalian stem-form. We may call this long-extinct root-form and its earliest descendants (a few genera of one family) “primitive mammals” or “stem-mammals” (Promammalia). As we have already seen, this root-form developed from the primitive Proreptile stem in a totally different direction from the birds, and soon separated from the main stem of the reptiles. The differences between the Mammals and the reptiles and birds are so important and characteristic that we can assume with complete confidence this division of the vertebrate stem at the commencement of the development of the Amniotes. The reptiles and birds, which we group together as the Sauropsids, generally agree in the characteristic structure of the skull and brain, and this is notably different from that of the Mammals. In most of the reptiles and birds the skull is connected with the first cervical vertebra (the atlas) by a single, and in the Mammals (and Amphibia) by a double, condyle at the back of the head. In the former the lower jaw is composed of several pieces, and connected with the skull so that it can move by a special maxillary bone (the quadratum); in the Mammals the lower jaw consists of one pair of bony pieces, which articulate directly with the temporal bone. Further, in the Sauropsids the skin is clothed with scales or feathers; in the Mammals with hair. The red blood-cells of the former have a nucleus; those of the latter have not. In fine, two quite characteristic features of the Mammals, which distinguish them not only from the birds and reptiles, but from all other animals, are the possession of a complete diaphragm and of mammary glands that produce the milk for the nutrition of the young. It is only in the Mammals that the diaphragm forms a transverse partition of the body-cavity, completely separating the pectoral from the abdominal cavity. It is only in the mammals that the mother suckles its young, and this rightly gives the name to the whole class (mamma = breast).
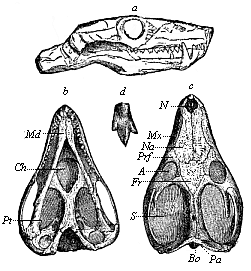
Fig. 267—Skull of a Triassic theromorphum (Galesaurus planiceps), from the Karoo formation in South Africa. (From Owen.) a from the right, b from below, c from above, d tricuspid tooth. N nostrils, Na nasal bone, Mx upper jaw, Prf prefrontal, Fr frontal bone, A eye-pits, S temple-pits. Pa Parietal eye, Bo joint at back of head, Pt pterygoid-bone, Md lower jaw.
From these pregnant facts of comparative anatomy and ontogeny it follows absolutely that the whole of the Mammals belong to a single natural stem, which branched off at an early date from the reptile-root. It follows further with the same absolute certainty that the human race is also a branch of this stem. Man shares all the characteristics I have described with all the Mammals, and differs in them from all other animals. Finally, from these facts we deduce with the same confidence those advances in the vertebrate organisation by which one branch of the Sauromammals was converted into the stem-form of the Mammals. Of these advances the chief were: (1) The characteristic modification of the skull and the brain; (2) the development of a hairy coat; (3) the complete formation of the diaphragm; and (4) the construction of the mammary glands and adaptation to suckling. Other important changes of structure proceeded step by step with these.
The epoch at which these important advances were made, and the foundation of the Mammal class was laid, may be put with great probability in the first section of the Mesozoic or secondary age—the Triassic period. The oldest fossil remains of mammals that we know were found in strata that belong to the earliest Triassic period—the upper Kueper. One of the earliest forms is the genus Dromatherium, from the North American Triassic (Fig. 268). Their teeth still strikingly recall those of the Pelycosauria. Hence we may assume that this small and probably insectivorous mammal belonged to the stem-group of the Promammals. We do not find any positive trace of the third and most advanced division of the Mammals—the Placentals. These (including man) are much younger, and we do not find indisputable fossil remains of them until the Cenozoic age, or the Tertiary period. This paleontological fact is very important, because it fully harmonises with the evolutionary succession of the Mammal orders that is deduced from their comparative anatomy and ontogeny.
The latter science teaches us that the whole Mammal class divides into three main groups or sub-classes, which correspond to three successive phylogenetic stages. These three stages, which also represent three important stages in our human genealogy, were first distinguished in 1816 by the eminent French zoologist, Blainville, and received the names of Ornithodelphia, Didelphia, and Monodelphia, according to the construction of the female organs (delphys = uterus or womb). Huxley afterwards gave them the names of Prototheria, Metatheria, and Epitheria. But the three sub-classes differ so widely from each other, not only in the construction of the sexual organs, but in many other respects also, that we may confidently draw up the following important phylogenetic thesis: The Monodelphia or Placentals descend from the Didelphia or Marsupials; and the latter, in turn, are descended from the Monotremes or Ornithodelphia.
Thus we must regard as the twenty-first stage in our genealogical tree the earliest and lowest chief group of the Mammals—the sub-class of the Monotremes (“cloaca-animals,” Ornithodelphia, or Prototheria, Figs. 269 and 270). They take their name from the cloaca which they share with all the lower Vertebrates. This cloaca is the common outlet for the passage of the excrements, the urine, and the sexual products. The urinary ducts and sexual canals open into the hindmost part of the gut, while in all the other Mammals they are separated from the rectum and anus. The latter have a special uro-genital outlet (porus urogenitalis). The bladder also opens into the cloaca in the Monotremes, and, indeed, apart from the two urinary ducts; in all the other Mammals the latter open directly into the bladder. It was proved by Haacke and Caldwell in 1884 that the Monotremes lay large eggs like the reptiles, while all the other Mammals are viviparous. In 1894 Richard Semon further proved that these large eggs, rich in food-yelk, have a partial segmentation and discoid gastrulation, as I had hypothetically assumed in 1879; here again they resemble their reptilian ancestors. The construction of the mammary gland is also peculiar in the Monotremes. In them the glands have no teats for the young animal to suck, but there is a special part of the breast pierced with holes like a sieve, from which the milk issues, and the young Monotreme must lick it off. Further, the brain of the Monotremes is very little advanced. It is feebler than that of any of the other Mammals. The fore-brain or cerebrum, in particular, is so small that it does not cover the cerebellum. In the skeleton (Fig. 270) the formation of the scapula among other parts is curious; it is quite different from that of the other Mammals, and rather agrees with that of the reptiles and Amphibia. Like these, the Monotremes have a strongly developed caracoideum. From these and other less prominent characteristics it follows absolutely that the Monotremes occupy the lowest place among the Mammals, and represent a transitional group between the Tocosauria and the rest of the Mammals. All these remarkable reptilian characters must have been possessed by the stem-form of the whole mammal class, the Promammal of the Triassic period, and have been inherited from the Proreptiles.
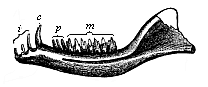
Fig. 268—Lower jaw of a Primitive Mammal or Promammal (Dromatherium silvestre) from the North American Triassic. i incisors, c canine, p premolars, m molars. (From Döderlein.)
During the Triassic and Jurassic periods the sub-class of the Monotremes was represented by a number of different stem-mammals. Numerous fossil remains of them have lately been discovered in the Mesozoic strata of Europe, Africa, and America. To-day there are only two surviving specimens of the group, which we place together in the family of the duck-bills, Ornithostoma. They are confined to Australia and the neighbouring island of Van Diemen’s Land (or Tasmania); they become scarcer every year, and will soon, like their blood-relatives, be counted among the extinct animals. One form lives in the rivers, and builds subterraneous dwellings on the banks; this is the Ornithorhyncus paradoxus, with webbed feet, a thick soft fur, and broad flat jaws, which look very much like the bill of a duck (Figs. 269, 270). The other form, the land duck-bill, or spiny ant-eater (Echidna hystrix), is very much like the anteaters in its habits and the peculiar construction of its thin snout and very long tongue; it is covered with needles, and can roll itself up like a hedgehog. A cognate form (Parechidna Bruyni) has lately been found in New Guinea.
These modern Ornithostoma are the scattered survivors of the vast Mesozoic group of Monotremes; hence they have the same interest in connection with the stem history of the Mammals as the living stem-reptiles (Hatteria) for that of the reptiles, and the isolated Acrania (Amphioxus) for the phylogeny of the Vertebrate stem.
The Australian duck-bills are distinguished externally by a toothless bird-like beak or snout. This absence of real bony teeth is a late result of adaptation, as in the toothless Placentals (Edentata, armadillos and ant-eaters). The extinct Monotremes, to which the Promammalia belonged, must have had developed teeth, inherited from the reptiles. Lately small rudiments of real molars have been discovered in the young of the Ornithorhyncus, which has horny plates in the jaws instead of real teeth.
The living Ornithostoma and the stem-forms of the Marsupials (or Didelphia) must be regarded as two widely diverging lines from the Promammals. This second sub-class of the Mammals is very interesting as a perfect intermediate stage between the other two. While the Marsupials retain a great part of the characteristics of the Monotremes, they have also acquired some of the chief features of the Placentals. Some features are also peculiar to the Marsupials, such as the construction of the male and female sexual organs and the form of the lower jaw. The Marsupials are distinguished by a peculiar hook-like bony process that bends from the corner of the lower jaw and points inwards. As most of the Placentals have not this process, we can, with some probability, recognise the Marsupial from this feature alone. Most of the mammal remains that we have from the Jurassic and Cretaceous deposits are merely lower jaws, and most of the jaws found in the Jurassic deposits at Stonesfield and Purbeck have the peculiar hook-like process that characterises the lower jaw of the Marsupial. On the strength of this paleontological fact, we may suppose that they belonged to Marsupials. Placentals do not seem to have existed at the middle of the Mesozoic age—not until towards its close (in the Cretaceous period). At all events, we have no fossil remains of indubitable Placentals from that period.
The existing Marsupials, of which the plant-eating kangaroo and the carnivorous opossum (Fig. 272) are the best known, differ a good deal in structure, shape, and size, and correspond in many respects to the various orders of Placentals. Most of them live in Australia, and a small part of the Australian and East Malayan islands. There is now not a single living Marsupial on the mainland of Europe, Asia, or Africa. It was very different during the Mesozoic and even during the Cenozoic age. The sedimentary deposits of these periods contain a great number and variety of marsupial remains, sometimes of a colossal size, in various parts of the earth, and even in Europe. We may infer from this that the existing Marsupials are the remnant of an extensive earlier group that was distributed all over the earth. It had to give way in the struggle for life to the more powerful Placentals during the Tertiary period. The survivors of the group were able to keep alive in Australia and South America because the one was completely separated from the other parts of the earth during the whole of the Tertiary period, and the other during the greater part of it.
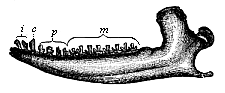
Fig. 271—Lower jaw of a Promammal (Dryolestes priscus), from the Jurassic of the Felsen strata. (From Marsh.)
From the comparative anatomy and ontogeny of the existing Marsupials we may draw very interesting conclusions as to their intermediate position between the earlier Monotremes and the later Placentals. The defective development of the brain (especially the cerebrum), the possession of marsupial bones, and the simple construction of the allantois (without any placenta as yet) were inherited by the Marsupials, with many other features, from the Monotremes, and preserved. On the other hand, they have lost the independent bone (caracoideum) at the shoulder-blade. But we have a more important advance in the disappearance of the cloaca; the rectum and anus are separated by a partition from the uro-genital opening (sinus urogenitalis). Moreover, all the Marsupials have teats on the mammary glands, at which the new-born animal sucks. The teats pass into the cavity of a pouch or pocket on the ventral side of the mother, and this is supported by a couple of marsupial bones. The young are born in a very imperfect condition, and carried by the mother for some time longer in her pouch, until they are fully developed (Fig. 272). In the giant kangaroo, which is as tall as a man, the embryo only develops for a month in the uterus, is then born in a very imperfect state, and finishes its growth in the mother’s pouch (marsupium); it remains in this about nine months, and at first hangs continually on to the teat of the mammary gland.
From these and other characteristics (especially the peculiar construction of the internal and external sexual organs in male and female) it is clear that we must conceive the whole sub-class of the Marsupials as one stem group, which has been developed from the Promammalia. From one branch of these Marsupials (possibly from more than one) the stem-forms of the higher Mammals, the Placentals, were afterwards evolved. Of the existing forms of the Marsupials, which have undergone various modifications through adaptation to different environments, the family of the opossums (Didelphida or Pedimana) seems to be the oldest and nearest to the common stem-form of the whole class. To this family belong the crab-eating opossum of Brazil (Fig. 272) and the opossum of Virginia, on the embryology of which Selenka has given us a valuable work (cf. Figs. 63–67 and 131–135). These Didelphida climb trees like the apes, grasping the branches with their hand-shaped hind feet. We may conclude from this that the stem-forms of the Primates, which we must regard as the earliest Lemurs, were evolved directly from the opossum. We must not forget, however, that the conversion of the five-toed foot into a prehensile hand is polyphyletic. By the same adaptation to climbing trees the habit of grasping their branches with the feet has in many different cases brought about that opposition of the thumb or great toe to the other toes which makes the hand prehensile. We see this in the climbing lizards (chameleon), the birds, and the tree-dwelling mammals of various orders.
Fig. 272—The crab-eating Opossum (Philander cancrivorus). The female has three young in the pouch. (From Brehm.)
Some zoologists have lately advanced the opposite opinion, that the Marsupials represent a completely independent sub-class of the Mammals, with no direct relation to the Placentals, and developing independently of them from the Monotremes. But this opinion is untenable if we examine carefully the whole organisation of the three sub-classes, and do not lay the chief stress on incidental features and secondary adaptations (such as the formation of the marsupium). It is then clear that the Marsupials—viviparous Mammals without placenta—are a necessary transition from the oviparous Monotremes to the higher Placentals with chorion-villi. In this sense the Marsupial class certainly contains some of man’s ancestors.
The long series of animal forms which we must regard as the ancestors of our race has been confined within narrower and narrower circles as our phylogenetic inquiry has progressed. The great majority of known animals do not fall in the line of our ancestry, and even within the vertebrate stem only a small number are found to do so. In the most advanced class of the stem, the mammals, there are only a few families that belong directly to our genealogical tree. The most important of these are the apes and their predecessors, the half-apes, and the earliest Placentals (Prochoriata).
The Placentals (also called Choriata, Monodelphia, Eutheria or Epitheria) are distinguished from the lower mammals we have just considered, the Monotremes and Marsupials, by a number of striking peculiarities. Man has all these distinctive features; that is a very significant fact. We may, on the ground of the most careful comparative-anatomical and ontogenetic research, formulate the thesis: “Man is in every respect a true Placental.” He has all the characteristics of structure and development that distinguish the Placentals from the two lower divisions of the mammals, and, in fact, from all other animals. Among these characteristics we must especially notice the more advanced development of the brain. The fore-brain or cerebrum especially is much more developed in them than in the lower animals. The corpus callosum, which forms a sort of wide bridge connecting the two hemispheres of the cerebrum, is only fully formed in the Placentals; it is very rudimentary in the Marsupials and Monotremes. It is true that the lowest Placentals are not far removed from the Marsupials in cerebral development; but within the placental group we can trace an unbroken gradation of progressive development of the brain, rising gradually from this lowest stage up to the elaborate psychic organ of the apes and man. The human soul—a physiological function of the brain—is in reality only a more advanced ape-soul.
The mammary glands of the Placentals are provided with teats like those of the Marsupials; but we never find in the Placentals the pouch in which the latter carry and suckle their young. Nor have they the marsupial bones in the ventral wall at the anterior border of the pelvis, which the Marsupials have in common with the Monotremes, and which are formed by a partial ossification of the sinews of the inner oblique abdominal muscle. There are merely a few insignificant remnants of them in some of the Carnivora. The Placentals are also generally without the hook-shaped process at the angle of the lower jaw which is found in the Marsupials.
However, the feature that characterises the Placentals above all others, and that has given its name to the whole sub-class, is the formation of the placenta. We have already considered the formation and significance of this remarkable embryonic organ when we traced the development of the chorion and the allantois in the human embryo (pp.165–9) The urinary sac or the allantois, the curious vesicle that grows out of the hind part of the gut, has essentially the same structure and function in the human embryo as in that of all the other Amniotes (cf. Figs. 194–6). There is a quite secondary difference, on which great stress has wrongly been laid, in the fact that in man and the higher apes the original cavity of the allantois quickly degenerates, and the rudiment of it sticks out as a solid projection from the primitive gut. The thin wall of the allantois consists of the same two layers or membranes as the wall of the gut—the gut-gland layer within and the gut-fibre layer without. In the gut-fibre layer of the allantois there are large blood-vessels, which serve for the nutrition, and especially the respiration, of the embryo—the umbilical vessels (p. 170).In the reptiles and birds the allantois enlarges into a spacious sac, which encloses the embryo with the amnion, and does not combine with the outer fœtal membrane (the chorion). This is the case also with the lowest mammals, the oviparous Monotremes and most of the Marsupials. It is only in some of the later Marsupials (Peramelida) and all the Placentals that the allantois develops into the distinctive and remarkable structure that we call the placenta.
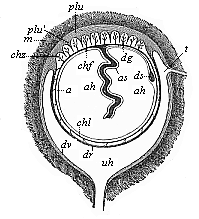
Fig. 273—Fœtal membranes of the human embryo (diagrammatic). m the thick muscular wall of the womb. plu placenta [the inner layer (plu′) of which penetrates into the chorion-villi (chz) with its processes]. chf tufted, chl smooth chorion. a amnion, ah amniotic cavity, as amniotic sheath of the umbilical cord (which passes under into the navel of the embryo—not given here), dg vitelline duct, ds yelk sac, dv, dr decidua (vera and reflexa). The uterine cavity (uh) opens below into the vagina and above on the right into an oviduct (t). (From Kölliker.)
The placenta is formed by the branches of the blood-vessels in the wall of the allantois growing into the hollow ectodermic tufts (villi) of the chorion, which run into corresponding depressions in the mucous membrane of the womb. The latter also is richly permeated with blood-vessels which bring the mother’s blood to the embryo. As the partition in the villi between the maternal blood-vessels and those of the fœtus is extremely thin, there is a direct exchange of fluid between the two, and this is of the greatest importance in the nutrition of the young mammal. It is true that the maternal vessels do not entirely pass into the fœtal vessels, so that the two kinds of blood are simply mixed. But the partition between them is so thin that the nutritive fluid easily transudes through it. By means of this transudation or diosmosis the exchange of fluids takes place without difficulty. The larger the embryo is in the placentals, and the longer it remains in the womb, the more necessary it is to have special structures to meet its great consumption of food.
In this respect there is a very conspicuous difference between the lower and higher mammals. In the Marsupials, in which the embryo is only a comparatively short time in the womb and is born in a very immature condition, the vascular arrangements in the yelk-sac and the allantois suffice for its nutrition, as we find them in the Monotremes, birds, and reptiles. But in the Placentals, where gestation lasts a long time, and the embryo reaches its full development under the protection of its enveloping membranes, there has to be a new mechanism for the direct supply of a large quantity of food, and this is admirably met by the formation of the placenta.
Branches of the blood-vessels penetrate into the chorion-villi from within, starting from the gut-fibre layer of the allantois, and bringing the blood of the fœtus through the umbilical vessels (Fig. 273 chz). On the other hand, a thick network of blood-vessels develops in the mucous membrane that clothes the inner surface of the womb, especially in the region of the depressions into which the chorion-villi penetrate (plu). This network of arteries contains maternal blood, brought by the uterine vessels. As the connective tissue between the enlarged capillaries of the uterus disappears, wide cavities filled with maternal blood appear, and into these the chorion-villi of the embryo penetrate. The sum of these vessels of both kinds, that are so intimately correlated at this point, together with the connective and enveloping tissue, is the placenta. The placenta consists, therefore, properly speaking, of two different though intimately connected parts—the fœtal placenta (Fig. 273 chz) within and the maternal or uterine placenta (plu) without. The latter is made up of the mucous coat of the uterus and its blood-vessels, the former of the tufted chorion and the umbilical vessels of the embryo (cf. Fig. 196).
Fig. 274—Skull of a fossil lemur (Adapis parisiensis), from the Miocene at Quercy. A lateral view from the right. B lower jaw, C lower molar, i incisors, c canines, p premolars, m molars.
The manner in which these two kinds of vessels combine in the placenta, and the structure, form, and size of it, differ a good deal in the various Placentals; to some extent they give us valuable data for the natural classification, and therefore the phylogeny, of the whole of this sub-class. On the ground of these differences we divide it into two principal sections; the lower Placentals or Indecidua, and the higher Placentals or Deciduata.
To the Indecidua belong three important groups of mammals: the Lemurs (Prosimiæ), the Ungulates (tapirs, horses, pigs, ruminants, etc.), and the Cetacea (dolphins and whales). In these Indecidua the villi are distributed over the whole surface of the chorion (or its greater part) either singly or in groups. They are only loosely connected with the mucous coat of the uterus, so that the whole fœtal membrane with its villi can be easily withdrawn from the uterine depressions like a hand from a glove. There is no real coalescence of the two placentas at any part of the surface of contact. Hence at birth the fœtal placenta alone comes away; the uterine placenta is not torn away with it.
The formation of the placenta is very different in the second and higher section of the Placentals, the Deciduata. Here again the whole surface of the chorion is thickly covered with the villi in the beginning. But they afterwards disappear from one part of the surface, and grow proportionately thicker on the other part. We thus get a differentiation between the smooth chorion (chorion laeve, Fig. 273 chl) and the thickly-tufted chorion (chorion frondosum, Fig. 273 chf). The former has only a few small villi or none at all; the latter is thickly covered with large and well-developed villi; this alone now constitutes the placenta. In the great majority of the Deciduata the placenta has the same shape as in man >(Figs. 197 and 200)—namely a thick, circular disk like a cake; so we find in the Insectivora, Chiroptera, Rodents, and Apes. This discoplacenta lies on one side of the chorion. But in the Sarcotheria (both the Carnivora and the seals, Pinnipedia) and in the elephant and several other Deciduates we find a zonoplacenta; in these the rich mass of villi runs like a girdle round the middle of the ellipsoid chorion, the two poles of it being free from them.
Still more characteristic of the Deciduates is the peculiar and very intimate connection between the chorion frondosum and the corresponding part of the mucous coat of the womb, which we must regard as a real coalescence of the two. The villi of the chorion push their branches into the blood-filled tissues of the coat of the uterus, and the vessels of each loop together so intimately that it is no longer possible to separate the fœtal from the maternal placenta; they form henceforth a compact and apparently simple placenta. In consequence of this coalescence, a whole piece of the lining of the womb comes away at birth with the fœtal membrane that is interlaced with it. This piece is called the “falling-away” membrane (decidua). It is also called the serous (spongy) membrane, because it is pierced like a sieve or sponge. All the higher Placentals that have this decidua are classed together as the “Deciduates.” The tearing away of the decidua at birth naturally causes the mother to lose a quantity of blood, which does not happen in the Indecidua. The last part of the uterine coat has to be repaired by a new growth after birth in the Deciduates. (Cf. Figs. 199, 200, pp. 168–70.)
In the various orders of the Deciduates, the placenta differs considerably both in outer form and internal structure. The extensive investigations of the last ten years have shown that there is more variation in these respects among the higher mammals than was formerly supposed. The physiological work of this important embryonic organ, the nutrition of the fœtus during its long sojourn in the womb, is accomplished in the various groups of the Placentals by very different and sometimes very elaborate structures. They have lately been fully described by Hans Strahl.
The phylogeny of the placenta has become more intelligible from the fact that we have found a number of transitional forms of it. Some of the Marsupials (Perameles) have the beginning of a placenta. In some of the Lemurs (Tarsius) a discoid placenta with decidua is developed.
While these important results of comparative embryology have been throwing further light on the close blood-relationship of man and the anthropoid apes in the last few years (p. 172), the great advance of paleontology has at the same time been affording us a deeper insight into the stem-history of the Placental group. In the seventh chapter of my Systematic Phylogeny of the Vertebrates I advanced the hypothesis that the Placentals form a single stem with many branches, which has been evolved from an older group of the Marsupials (Prodidelphia). The four great legions of the Placentals—Rodents, Ungulates, Carnassia, and Primates—are sharply separated to-day by important features of organisation. But if we consider their extinct ancestors of the Tertiary period, the differences gradually disappear, the deeper we go in the Cenozoic deposits; in the end we find that they vanish altogether. The primitive stem-forms of the Rodents (Esthonychida), the Ungulates (Chondylarthra), the Carnassia (Ictopsida), and the Primates (Lemuravida) are so closely related at the beginning of the Tertiary period that we might group them together as different families of one order, the Proplacentals (Mallotheria or Prochoriata).
Hence the great majority of the Placentals have no direct and close relationship to man, but only the legion of the Primates. This is now generally divided into three orders—the half-apes (Prosimiæ), apes (Simiæ), and man (Anthropi). The lemurs or half-apes are the stem-group, descending from the older Mallotheria of the Cretaceous period. From them the apes were evolved in the Tertiary period, and man was formed from these towards its close.
The Lemurs (Prosimiæ) have few living representatives. But they are very interesting, and are the last survivors of a once extensive group. We find many fossil remains of them in the older Tertiary deposits of Europe and North America, in the Eocene and Miocene. We distinguish two sub-orders, the fossil Lemuravida and the modern Lemurogona. The earliest and most primitive forms of the Lemuravida are the Pachylemurs (Hypopsodina); they come next to the earliest Placentals (Prochoriata), and have the typical full dentition, with forty-four teeth (3.1.4.3. over 3.1.4.3.). The Necrolemurs (Adapida, Fig. 274) have only forty teeth, and have lost an incisor in each jaw (2.1.4.3. over 2.1.4.3.). The dentition is still further reduced in the Lemurogona (Autolemures), which usually have only thirty-six teeth (2.1.3.3. over 2.1.3.3.). These living survivors are scattered far over the southern part of the Old World. Most of the species live in Madagascar, some in the Sunda Islands, others on the mainland of Asia and Africa. They are gloomy and melancholic animals; they live a quiet life, climbing trees, and eating fruit and insects. They are of different kinds. Some are closely related to the Marsupials (especially the opossum). Others (Macrotarsi) are nearer to the Insectivora, others again (Chiromys) to the Rodents. Some of the lemurs (Brachytarsi) approach closely to the true apes. The numerous fossil remains of half-apes and apes that have been recently found in the Tertiary deposits justify us in thinking that man’s ancestors were represented by several different species during this long period. Some of these were almost as big as men, such as the diluvial lemurogonon Megaladapis of Madagascar.
Next to the lemurs come the true apes (Simiæ), the twenty-sixth stage in our ancestry. It has been beyond question for some time now that the apes approach nearest to man in every respect of all the animals. Just as the lowest apes come close to the lemurs, so the highest come next to man. When we carefully study the comparative anatomy of the apes and man, we can trace a gradual and uninterrupted advance in the organisation of the ape up to the purely human frame, and, after impartial examination of the “ape problem” that has been discussed of late years with such passionate interest, we come infallibly to the important conclusion, first formulated by Huxley in 1863: “Whatever systems of organs we take, the comparison of their modifications in the series of apes leads to the same result: that the anatomic differences that separate man from the gorilla and chimpanzee are not as great as those that separate the gorilla from the lower apes.” Translated into phylogenetic language, this “pithecometra-law,” formulated in such masterly fashion by Huxley, is quite equivalent to the popular saying: “Man is descended from the apes.”
In the very first exposition of his profound natural classification (1735) Linné placed the anthropoid mammals at the head of the animal kingdom, with three genera: man, the ape, and the sloth. He afterwards called them the “Primates”—the “lords” of the animal world; he then also separated the lemur from the true ape, and rejected the sloth. Later zoologists divided the order of Primates. First the Gottingen anatomist, Blumenbach, founded a special order for man, which he called Bimana (“two-handed”); in a second order he united the apes and lemurs under the name of Quadrumana (“four-handed”); and a third order was formed of the distantly-related Chiroptera (bats, etc.). The separation of the Bimana and Quadrumana was retained by Cuvier and most of the subsequent zoologists. It seems to be extremely important, but, as a matter of fact, it is totally wrong. This was first shown in 1863 by Huxley, in his famous Man’s Place in Nature. On the strength of careful comparative anatomical research he proved that the apes are just as truly “two-handed” as man; or, if we prefer to reverse it, that man is as truly four-handed as the ape. He showed convincingly that the ideas of hand and foot had been wrongly defined, and had been improperly based on physiological instead of morphological grounds. The circumstance that we oppose the thumb to the other four fingers in our hand, and so can grasp things, seemed to be a special distinction of the hand in contrast to the foot, in which the corresponding great toe cannot be opposed in this way to the others. But the apes can grasp with the hind-foot as well as the fore, and so were regarded as quadrumanous. However, the inability to grasp that we find in the foot of civilised man is a consequence of the habit of clothing it with tight coverings for thousands of years. Many of the bare-footed lower races of men, especially among the negroes, use the foot very freely in the same way as the hand. As a result of early habit and continued practice, they can grasp with the foot (in climbing trees, for instance) just as well as with the hand. Even new-born infants of our own race can grasp very strongly with the great toe, and hold a spoon with it as firmly as with the hand. Hence the physiological distinction between hand and foot can neither be pressed very far, nor has it a scientific basis. We must look to morphological characters.
As a matter of fact, it is possible to draw such a sharp morphological distinction—a distinction based on anatomic structure—between the fore and hind extremity. In the formation both of the bony skeleton and of the muscles that are connected with the hand and foot before and behind there are material and constant differences; and these are found both in man and the ape. For instance, the number and arrangement of the smaller bones of the hand and foot are quite different. There are similar constant differences in the muscles. The hind extremity always has three muscles (a short flexor muscle, a short extensor muscle, and a long calf-muscle) that are not found in the fore extremity. The arrangement of the muscles also is different before and behind. These characteristic differences between the fore and hind extremities are found in man as well as the ape. There can be no doubt, therefore, that the ape’s foot deserves that name just as much as the human foot does, and that all true apes are just as “bimanous” as man. The common distinction of the apes as “quadrumanous” is altogether wrong morphologically.
But it may be asked whether, quite apart from this, we can find any other features that distinguish man more sharply from the ape than the various species of apes are distinguished from each other. Huxley gave so complete and demonstrative a reply to this question that the opposition still raised on many sides is absolutely without foundation. On the ground of careful comparative anatomical research, Huxley proved that in all morphological respects the differences between the highest and lowest apes are greater than the corresponding differences between the highest apes and man. He thus restored Linné’s order of the Primates (excluding the bats), and divided it into three sub-orders, the first composed of the half-apes (Lemuridæ), the second of the true apes (Simiadæ), the third of men (Anthropidæ).
But, as we wish to proceed quite consistently and impartially on the laws of systematic logic, we may, on the strength of Huxley’s own law, go a good deal farther in this division. We are justified in going at least one important step farther, and assigning man his natural place within one of the sections of the order of apes. All the features that characterise this group of apes are found in man, and not found in the other apes. We do not seem to be justified, therefore, in founding for man a special order distinct from the apes.
The order of the true apes (Simiæ or Pitheca)—excluding the lemurs—has long been divided into two principal groups, which also differ in their geographical distribution. One group (Hesperopitheca, or western apes) live in America. The other group, to which man belongs, are the Eopitheca or eastern apes; they are found in Asia and Africa, and were formerly in Europe. All the eastern apes agree with man in the features that are chiefly used in zoological classification to distinguish between the two simian groups, especially in the dentition. The objection might be raised that the teeth are too subordinate an organ physiologically for us to lay stress on them in so important a question. But there is a good reason for it; it is with perfect justice that zoologists have for more than a century paid particular attention to the teeth in the systematic division and arrangement of the orders of mammals. The number, form, and arrangement of the teeth are much more faithfully inherited in the various orders than most other characters.
Hence the form of dentition in man is very important. In the fully developed condition we have thirty-two teeth; of these eight are incisors, four canine, and twenty molars. The eight incisors, in the middle of the jaws, have certain characteristic differences above and below. In the upper jaw the inner incisors are larger than the outer; in the lower jaw the inner are the smaller. Next to these, at each side of both jaws, is a canine (or “eye tooth”), which is larger than the incisors. Sometimes it is very prominent in man, as it is in most apes and many of the other mammals, and forms a sort of tusk. Next to this there are five molars above and below on each side, the first two of which (the “pre-molars”) are small, have only one root, and are included in the change of teeth; the three back ones are much larger, have two roots, and only come with the second teeth. The apes of the Old World, or all the living or fossil apes of Asia, Africa, and Europe, have the same dentition as man.
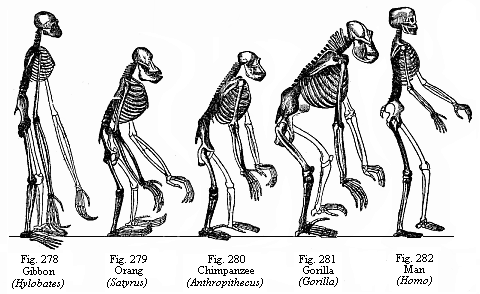
Fig. 278–282—Skeletons of a man and the four
anthropoid apes.
(Fig. 278, Gibbon; Fig. 279, Orang; Fig. 280,
Chimpanzee; Fig. 281, Gorilla; Fig. 282, Man.
(From Huxley.) Cf.
Figs. 203–209.
On the other hand, all the American apes have an additional pre-molar in each half of the jaw. They have six molars above and below on each side, or thirty-six teeth altogether. This characteristic difference between the eastern and western apes has been so faithfully inherited that it is very instructive for us. It is true that there seems to be an exception in the case of a small family of South American apes. The small silky apes (Arctopitheca or Hapalidæ), which include the tamarin (Midas) and the brush-monkey (Jacchus), have only five molars in each half of the jaw (instead of six), and so seem to be nearer to the eastern apes. But it is found, on closer examination, that they have three premolars, like all the western apes, and that only the last molar has been lost. Hence the apparent exception really confirms the above distinction.
Of the other features in which the two groups of apes differ, the structure of the nose is particularly instructive and conspicuous. All the eastern apes have the same type of nose as man—a comparatively narrow partition between the two halves, so that the nostrils run downwards. In some of them the nose protrudes as far as in man, and has the same characteristic structure. We have already alluded to the curious long-nosed apes, which have a long, finely-curved nose. Most of the eastern apes have, it is true, rather flat noses, like, for instance, the white-nosed monkey (Fig. 276); but the nasal partition is thin and narrow in them all. The American apes have a different type of nose. The partition is very broad and thick at the bottom, and the wings of the nostrils are not developed, so that they point outwards instead of downwards. This difference in the form of the nose is so constantly inherited in both groups that the apes of the New World are called “flat-nosed” (Platyrrhinæ), and those of the Old World “narrow-nosed” (Catarrhinæ). The bony passage of the ear (at the bottom of which is the tympanum) is short and wide in all the Platyrrhines, but long and narrow in all the Catarrhines; and in man this difference also is significant.
This division of the apes into Platyrrhines and Catarrhines, on the ground of the above hereditary features, is now generally admitted in zoology, and receives strong support from the geographical distribution of the two groups in the east and west. It follows at once, as regards the phylogeny of the apes, that two divergent lines proceeded from the common stem-form of the ape-order in the early Tertiary period, one of which spread over the Old, the other over the New, World. It is certain that all the Platyrrhines come of one stock, and also all the Catarrhines; but the former are phylogenetically older, and must be regarded as the stem-group of the latter.
What can we deduce from this with regard to our own genealogy? Man has just the same characters, the same form of dentition, auditory passage, and nose, as all the Catarrhines; in this he radically differs from the Platyrrhines. We are thus forced to assign him a position among the eastern apes in the order of Primates, or at least place him alongside of them. But it follows that man is a direct blood relative of the apes of the Old World, and can be traced to a common stem-form together with all the Catarrhines. In his whole organisation and in his origin man is a true Catarrhine; he originated in the Old World from an unknown, extinct group of the eastern apes. The apes of the New World, or the Platyrrhines, form a divergent branch of our genealogical tree, and this is only distantly related at its root to the human race. We must assume, of course, that the earliest Eocene apes had the full dentition of the Platyrrhines; hence we may regard this stem-group as a special stage (the twenty-sixth) in our ancestry, and deduce from it (as the twenty-seventh stage) the earliest Catarrhines.
We have now reduced the circle of our nearest relatives to the small and comparatively scanty group that is represented by the sub-order of the Catarrhines; and we are in a position to answer the question of man’s place in this sub-order, and say whether we can deduce anything further from this position as to our immediate ancestors. In answering this question the comprehensive and able studies that Huxley gives of the comparative anatomy of man and the various Catarrhines in his Man’s Place in Nature are of great assistance to us. It is quite clear from these that the differences between man and the highest Catarrhines (gorilla, chimpanzee, and orang) are in every respect slighter than the corresponding differences between the highest and the lowest Catarrhines (white-nosed monkey, macaco, baboon, etc.). In fact, within the small group of the tail-less anthropoid apes the differences between the various genera are not less than the differences between them and man. This is seen by a glance at the skeletons that Huxley has put together (Figs. 278–282). Whether we take the skull or the vertebral column or the ribs or the fore or hind limbs, or whether we extend the comparison to the muscles, blood-vessels, brain, placenta, etc., we always reach the same result on impartial examination—that man is not more different from the other Catarrhines than the extreme forms of them (for instance, the gorilla and baboon) differ from each other. We may now, therefore, complete the Huxleian law we have already quoted with the following thesis: “Whatever system of organs we take, a comparison of their modifications in the series of Catarrhines always leads to the same conclusion; the anatomic differences that separate man from the most advanced Catarrhines (orang, gorilla, chimpanzee) are not as great as those that separate the latter from the lowest Catarrhines (white-nosed monkey, macaco, baboon).”
We must, therefore, consider the descent of man from other Catarrhines to be fully proved. Whatever further information on the comparative anatomy and ontogeny of the living Catarrhines we may obtain in the future, it cannot possibly disturb this conclusion. Naturally, our Catarrhine ancestors must have passed through a long series of different forms before the human type was produced. The chief advances that effected this “creation of man,” or his differentiation from the nearest related Catarrhines, were: the adoption of the erect posture and the consequent greater differentiation of the fore and hind limbs, the evolution of articulate speech and its organ, the larynx, and the further development of the brain and its function, the soul; sexual selection had a great influence in this, as Darwin showed in his famous work.
With an eye to these advances we can distinguish at least four important stages in our simian ancestry, which represent prominent points in the historical process of the making of man. We may take, after the Lemurs, the earliest and lowest Platyrrhines of South America, with thirty-six teeth, as the twenty-sixth stage of our genealogy; they were developed from the Lemurs by a peculiar modification of the brain, teeth, nose, and fingers. From these Eocene stem-apes were formed the earliest Catarrhines or eastern apes, with the human dentition (thirty-two teeth), by modification of the nose, lengthening of the bony channel of the ear, and the loss of four pre-molars. These oldest stem-forms of the whole Catarrhine group were still thickly coated with hair, and had long tails—baboons (Cynopitheca) or tailed apes (Menocerca, Fig. 276). They lived during the Tertiary period, and are found fossilised in the Miocene. Of the actual tailed apes perhaps the nearest to them are the Semnopitheci.
If we take these Semnopitheci as the twenty-seventh stage in our ancestry, we may put next to them, as the twenty-eighth, the tail-less anthropoid apes. This name is given to the most advanced and man-like of the existing Catarrhines. They were developed from the other Catarrhines by losing the tail and part of the hair, and by a higher development of the brain, which found expression in the enormous growth of the skull. Of this remarkable family there are only a few genera to-day, and we have already dealt with them (Chapter XV)—the gibbon (Hylobates, Fig. 203) and orang (Satyrus, Figs. 204, 205) in South-Eastern Asia and the Archipelago; and the chimpanzee (Anthropithecus, Figs. 206, 207) and gorilla (Gorilla, Fig. 208) in Equatorial Africa.
The great interest that every thoughtful man takes in these nearest relatives of ours has found expression recently in a fairly large literature. The most distinguished of these works for impartial treatment of the question of affinity is Robert Hartmann’s little work on The Anthropoid Apes. Hartmann divides the primate order into two families: (1) Primarii (man and the anthropoid apes); and (2) Simianæ (true apes, Catarrhines and Platyrrhines). Professor Klaatsch, of Heidelberg, has advanced a different view in his interesting and richly illustrated work on The Origin and Development of the Human Race. This is a substantial supplement to my Anthropogeny, in so far as it gives the chief results of modern research on the early history of man and civilisation. But when Klaatsch declares the descent of man from the apes to be “irrational, narrow-minded, and false,” in the belief that we are thinking of some living species of ape, we must remind him that no competent scientist has ever held so narrow a view. All of us look merely—in the sense of Lamarck and Darwin—to the original unity (admitted by Klaatsch) of the primate stem. This common descent of all the Primates (men, apes, and lemurs) from one primitive stem-form, from which the most far-reaching conclusions follow for the whole of anthropology and philosophy, is admitted by Klaatsch as well as by myself and all other competent zoologists who accept the theory of evolution in general. He says explicitly (p. 172): “The three anthropoid apes—gorilla, chimpanzee, and orang—seem to be branches from a common root, and this was not far from that of the gibbon and man.” That is in the main the opinion that I have maintained (especially against Virchow) in a number of works ever since 1866. The hypothetical common ancestor of all the Primates, which must have lived in the earliest Tertiary period (more probably in the Cretaceous), was called by me Archiprimus; Klaatsch now calls it Primatoid. Dubois has proposed the appropriate name of Prothylobates for the common and much younger stem-form of the anthropomorpha (man and the anthropoid apes). The actual Hylobates is nearer to it than the other three existing anthropoids. None of these can be said to be absolutely the most man-like. The gorilla comes next to man in the structure of the hand and foot, the chimpanzee in the chief features of the skull, the orang in brain development, and the gibbon in the formation of the chest. None of these existing anthropoid apes is among the direct ancestors of our race; they are scattered survivors of an ancient branch of the Catarrhines, from which the human race developed in a particular direction.
Although man is directly connected with this anthropoid family and originates from it, we may assign an important intermediate form between the Prothylobates and him (the twenty-ninth stage in our ancestry), the ape-men (Pithecanthropi). I gave this name in the History of Creation to the “speechless primitive men” (Alali), which were men in the ordinary sense as far as the general structure is concerned (especially in the differentiation of the limbs), but lacked one of the chief human characteristics, articulate speech and the higher intelligence that goes with it, and so had a less developed brain. The phylogenetic hypothesis of the organisation of this “ape-man” which I then advanced was brilliantly confirmed twenty-four years afterwards by the famous discovery of the fossil Pithecanthropus erectus by Eugen Dubois (then military surgeon in Java, afterwards professor at Amsterdam). In 1892 he found at Trinil, in the residency of Madiun in Java, in Pliocene deposits, certain remains of a large and very man-like ape (roof of the skull, femur, and teeth), which he described as “an erect ape-man” and a survivor of a “stem-form of man” (Fig. 283). Naturally, the Pithecanthropus excited the liveliest interest, as the long-sought transitional form between man and the ape: we seemed to have found “the missing link.” There were very interesting scientific discussions of it at the last three International Congresses of Zoology (Leyden, 1895, Cambridge, 1898, and Berlin, 1901). I took an active part in the discussion at Cambridge, and may refer the reader to the paper I read there on “The Present Position of Our Knowledge of the Origin of Man” (translated by Dr. Gadow with the title of The Last Link).
An extensive and valuable literature has grown up in the last ten years on the Pithecanthropus and the pithecoid theory connected with it. A number of distinguished anthropologists, anatomists, paleontologists, and phylogenists have taken part in the controversy, and made use of the important data furnished by the new science of pre-historic research. Hermann Klaatsch has given a good summary of them, with many fine illustrations, in the above-mentioned work. I refer the reader to it as a valuable supplement to the present work, especially as I cannot go any further here into these anthropological and pre-historic questions. I will only repeat that I think he is wrong in the attitude of hostility that he affects to take up with regard to my own views on the descent of man from the apes.
The most powerful opponent of the pithecoid theory—and the theory of evolution in general—during the last thirty years (until his death in September, 1902) was the famous Berlin anatomist, Rudolf Virchow. In the speeches which he delivered every year at various congresses and meetings on this question, he was never tired of attacking the hated “ape theory.” His constant categorical position was: “It is quite certain that man does not descend from the ape or any other animal.” This has been repeated incessantly by opponents of the theory, especially theologians and philosophers. In the inaugural speech that he delivered in 1894 at the Anthropological Congress at Vienna, he said that “man might just as well have descended from a sheep or an elephant as from an ape.” Absurd expressions like this only show that the famous pathological anatomist, who did so much for medicine in the establishment of cellular pathology, had not the requisite attainments in comparative anatomy and ontogeny, systematic zoology and paleontology, for sound judgment in the province of anthropology. The Strassburg anatomist, Gustav Schwalbe, deserved great praise for having the moral courage to oppose this dogmatic and ungrounded teaching of Virchow, and showing its untenability. The recent admirable works of Schwalbe on the Pithecanthropus, the earliest races of men, and the Neanderthal skull (1897–1901) will supply any candid and judicious reader with the empirical material with which he can convince himself of the baselessness of the erroneous dogmas of Virchow and his clerical friends (J. Ranke, J. Bumüller, etc.).
As the Pithecanthropus walked erect, and his brain (judging from the capacity of his skull, Fig. 283) was midway between the lowest men and the anthropoid apes, we must assume that the next great step in the advance from the Pithecanthropus to man was the further development of human speech and reason.
Comparative philology has recently shown that human speech is polyphyletic in origin; that we must distinguish several (probably many) different primitive tongues that were developed independently. The evolution of language also teaches us (both from its ontogeny in the child and its phylogeny in the race) that human speech proper was only gradually developed after the rest of the body had attained its characteristic form. It is probable that language was not evolved until after the dispersal of the various species and races of men, and this probably took place at the commencement of the Quaternary or Diluvial period. The speechless ape-men or Alali certainly existed towards the end of the Tertiary period, during the Pliocene, possibly even the Miocene, period.
The third, and last, stage of our animal ancestry is the true or speaking man (Homo), who was gradually evolved from the preceding stage by the advance of animal language into articulate human speech. As to the time and place of this real “creation of man” we can only express tentative opinions. It was probably during the Diluvial period in the hotter zone of the Old World, either on the mainland in tropical Africa or Asia or on an earlier continent (Lemuria—now sunk below the waves of the Indian Ocean), which stretched from East Africa (Madagascar, Abyssinia) to East Asia (Sunda Islands, Further India). I have given fully in my History of Creation, (chapter xxviii) the weighty reasons for claiming this descent of man from the anthropoid eastern apes, and shown how we may conceive the spread of the various races from this “Paradise” over the whole earth. I have also dealt fully with the relations of the various races and species of men to each other.
First Stage: The Protists
Man’s ancestors are unicellular protozoa, originally unnucleated Monera like the Chromacea, structureless green particles of plasm; afterwards real nucleated cells (first plasmodomous Protophyta, like the Palmella; then plasmophagous Protozoa, like the Amœba).
Second Stage: The Blastæads
Man’s ancestors are round cœnobia or colonies of Protozoa; they consist of a close association of many homogeneous cells, and thus are individuals of the second order. They resemble the round cell-communities of the Magospheræ and Volvocina, equivalent to the ontogenetic blastula: hollow globules, the wall of which consists of a single layer of ciliated cells (blastoderm).
Third Stage: The Gastræads
Man’s ancestors are Gastræads, like the simplest of the actual Metazoa (Prophysema, Olynthus, Hydra, Pemmatodiscus). Their body consists merely of a primitive gut, the wall of which is made up of the two primary germinal layers.
Fourth Stage: The Platodes
Man’s ancestors have substantially the organisation of simple Platodes (at first like the cryptocœlic Platodaria, later like the rhabdocœlic Turbellaria). The leaf-shaped bilateral-symmetrical body has only one gut-opening, and develops the first trace of a nervous centre from the ectoderm in the middle line of the back (Figs. 239, 240).
Fifth Stage: The Vermalia
Man’s ancestors have substantially the organisation of unarticulated Vermalia, at first Gastrotricha (Ichthydina), afterwards Frontonia (Nemertina, Enteropneusta). Four secondary germinal layers develop, two middle layers arising between the limiting layers (cœloma). The dorsal ectoderm forms the vertical plate, acroganglion (Fig. 243).
Sixth Stage: The Prochordonia
Man’s ancestors have substantially the organisation of a simple unarticulated Chordonium (Copelata and Ascidia-larvæ). The unsegmented chorda develops between the dorsal medullary tube and the ventral gut-tube. The simple cœlom-pouches divide by a frontal septum into two on each side; the dorsal pouch (episomite) forms a muscle-plate; the ventral pouch (hyposomite) forms a gonad. Head-gut with gill-clefts.
Seventh Stage: The Acrania
Man’s ancestors are skull-less Vertebrates, like the Amphioxus. The body is a series of metamera, as several of the primitive segments are developed. The head contains in the ventral half the branchial gut, the trunk the hepatic gut. The medullary tube is still simple. No skull, jaws, or limbs.
Eighth Stage: The Cyclostoma
Man’s ancestors are jaw-less Craniotes (like the Myxinoida and Petromyzonta). The number of metamera increases. The fore-end of the medullary tube expands into a vesicle and forms the brain, which soon divides into five cerebral vesicles. In the sides of it appear the three higher sense-organs: nose, eyes, and auditory vesicles. No jaws, limbs, or floating bladder.
Ninth Stage: The Ichthyoda
Man’s ancestors are fish-like Craniotes: (1) Primitive fishes (Selachii); (2) plated fishes (Ganoida); (3) amphibian fishes (Dipneusta); (4) mailed amphibia (Stegocephala). The ancestors of this series develop two pairs of limbs: a pair of fore (breast-fins) and of hind (belly-fins) legs. The gill-arches are formed between the gill-clefts: the first pair form the maxillary arches (the upper and lower jaws). The floating bladder (lung) and pancreas grow out of the gut.
Tenth Stage: The Amniotes
Man’s ancestors are Amniotes or gill-less Vertebrates: (1) Primitive Amniotes (Proreptilia); (2) Sauromammals; (3) Primitive Mammals (Monotremes); (4) Marsupials; (5) Lemurs (Prosimiæ); (6) Western apes (Platyrrhinæ); (7) Eastern apes (Catarrhinæ): at first tailed Cynopitheca; then tail-less anthropoids; later speechless ape-men (Alali); finally speaking man. The ancestors of these Amniotes develop an amnion and allantois, and gradually assume the mammal, and finally the specifically human, form.
The previous chapters have taught us how the human body as a whole develops from the first simple rudiment, a single layer of cells. The whole human race owes its origin, like the individual man, to a simple cell. The unicellular stem-form of the race is reproduced daily in the unicellular embryonic stage of the individual. We have now to consider in detail the evolution of the various parts that make up the human frame. I must, naturally, confine myself to the most general and principal outlines; to make a special study of the evolution of each organ and tissue is both beyond the scope of this work, and probably beyond the anatomic capacity of most of my readers to appreciate. In tracing the evolution of the various organs we shall follow the method that has hitherto guided us, except that we shall now have to consider the ontogeny and phylogeny of the organs together. We have seen, in studying the evolution of the body as a whole, that phylogeny casts a light over the darker paths of ontogeny, and that we should be almost unable to find our way in it without the aid of the former. We shall have the same experience in the study of the organs in detail, and I shall be compelled to give simultaneously their ontogenetic and phylogenetic origin. The more we go into the details of organic development, and the more closely we follow the rise of the various parts, the more we see the inseparable connection of embryology and stem-history. The ontogeny of the organs can only be understood in the light of their phylogeny, just as we found of the embryology of the whole body. Each embryonic form is determined by a corresponding stem-form. This is true of details as well as of the whole.
We will consider first the animal and then the vegetal systems of organs of the body. The first group consists of the psychic and the motor apparatus. To the former belong the skin, the nervous system, and the sense-organs. The motor apparatus is composed of the passive and the active organs of movement (the skeleton and the muscles). The second or vegetal group consists of the nutritive and the reproductive apparatus. To the nutritive apparatus belong the alimentary canal with all its appendages, the vascular system, and the renal (kidney) system. The reproductive apparatus comprises the different organs of sex (embryonic glands, sexual ducts, and copulative organs).
As we know from previous chapters (XI–XIII), the animal systems of organs (the organs of sensation and presentation) develop for the most part out of the outer primary germ-layer, or the cutaneous (skin) layer. On the other hand, the vegetal systems of organs arise for the most part from the inner primary germ-layer, the visceral layer. It is true that this antithesis of the animal and vegetal spheres of the body in man and all the higher animals is by no means rigid; several parts of the animal apparatus (for instance, the greater part of the muscles) are formed from cells that come originally from the entoderm; and a great part of the vegetative apparatus (for instance, the mouth-cavity and the gonoducts) are composed of cells that come from the ectoderm.
In the more advanced animal body there is so much interlacing and displacement of the various parts that it is often very difficult to indicate the sources of them. But, broadly speaking, we may take it as a positive and important fact that in man and the higher animals the chief part of the animal organs comes from the ectoderm, and the greater part of the vegetative organs from the entoderm. It was for this reason that Carl Ernst von Baer called the one the animal and the other the vegetative layer (see p. 16).
The solid foundation of this important thesis is the gastrula, the most instructive embryonic form in the animal world, which we still find in the same shape in the most diverse classes of animals. This form points demonstrably to a common stem-form of all the Metazoa, the Gastræa; in this long-extinct stem-form the whole body consisted throughout life of the two primary germinal layers, as is now the case temporarily in the gastrula; in the Gastræa the simple cutaneous (skin) layer actually represented all the animal organs and functions, and the simple visceral (gut) layer all the vegetal organs and functions. This is the case with the modern Gastræads (Fig. 233); and it is also the case potentially with the gastrula.
We shall easily see that the gastræa theory is thus able to throw a good deal of light, both morphologically and physiologically, on some of the chief features of embryonic development, if we take up first the consideration of the chief element in the animal sphere, the psychic apparatus or sensorium and its evolution. This apparatus consists of two very different parts, which seem at first to have very little connection with each other—the outer skin, with all its hairs, nails, sweat-glands, etc., and the nervous system. The latter comprises the central nervous system (brain and spinal cord), the peripheral, cerebral, and spinal nerves, and the sense-organs. In the fully-formed vertebrate body these two chief elements of the sensorium lie far apart, the skin being external to, and the central nervous system in the very centre of, the body. The one is only connected with the other by a section of the peripheral nervous system and the sense-organs. Nevertheless, as we know from human embryology, the medullary tube is formed from the cutaneous layer. The organs that discharge the most advanced functions of the animal body—the organs of the soul, or of psychic life—develop from the external skin. This is a perfectly natural and necessary process. If we reflect on the historical evolution of the psychic and sensory functions, we are forced to conclude that the cells which accomplish them must originally have been located on the outer surface of the body. Only elementary organs in this superficial position could directly receive the influences of the environment. Afterwards, under the influence of natural selection, the cellular group in the skin which was specifically “sensitive” withdrew into the inner and more protected part of the body, and formed there the foundation of a central nervous organ. As a result of increased differentiation, the skin and the central nervous system became further and further separated, and in the end the two were only permanently connected by the afferent peripheral sensory nerves.
Fig. 284—The human skin in vertical section (from Ecker), highly magnified, a horny layer of the epidermis, b mucous layer of the epidermis, c papillæ of the corium, d blood-vessels of same, ef ducts of the sweat-glands (g), h fat-glands in the corium, i nerve, passing into a tactile corpuscle above.
The observations of the comparative anatomist are in complete accord with this view. He tells us that large numbers of the lower animals have no nervous system, though they exercise the functions of sensation and will like the higher animals. In the unicellular Protozoa, which do not form germinal layers, there is, of course, neither nervous system nor skin. But in the second division of the animal kingdom also, the Metazoa, there is at first no nervous system. Its functions are represented by the simple cell-layer of the ectoderm, which the lower Metazoa have inherited from the Gastræa (Fig. 30 e). We find this in the lowest Zoophytes—the Gastræads, Physemaria, and Sponges (Figs. 233–238). The lowest Cnidaria (the hydroid polyps) also are little superior to the Gastræads in structure. Their vegetative functions are accomplished by the simple visceral layer, and their animal functions by the simple cutaneous layer. In these cases the simple cell-layer of the ectoderm is at once skin, locomotive apparatus, and nervous system.
When we come to the higher Metazoa, in which the sensory functions and their organs are more advanced, we find a division of labour among the ectodermic cells. Groups of sensitive nerve cells separate from the ordinary epidermic cells; they retire into the more protected tissue of the mesodermic under-skin, and form special neural ganglia there. Even in the Platodes, especially the Turbellaria, we find an independent nervous system, which has separated from the outer skin. This is the “upper pharyngeal ganglion,” or acroganglion, situated above the gullet (Fig. 241 g).From this rudimentary structure has been developed the elaborate central nervous system of the higher animals. In some of the higher worms, such as the earth-worm, the first rudiment of the central nervous system (Fig. 74 n) is a local thickening of the skin-sense layer (hs), which afterwards separates altogether from the horny plate. In the earliest Platodes (Cryptocœla) and Vermalia (Gastrotricha) the acroganglion remains in the epidermis. But the medullary tube of the Vertebrates originates in the same way. Our embryology has taught us that this first structure of the central nervous system also develops originally from the outer germinal layer.
Let us now examine more closely the evolution of the human skin, with its various appendages, the hairs and glands. This external covering has, physiologically, a double and important part to play. It is, in the first place, the common integument that covers the whole surface of the body, and forms a protective envelope for the other organs. As such it also effects a certain exchange of matter between the body and the surrounding atmosphere (exhalation, perspiration). In the second place, it is the earliest and original sense organ, the common organ of feeling that experiences the sensation of the temperature of the environment and the pressure or resistance of bodies that come into contact.
The human skin (like that of all the higher animals) is composed of two layers, the outer and the inner or underlying skin. The outer skin or epidermis, consists of simple ectodermic cells, and contains no blood-vessels (Fig. 284 a, b). It develops from the outer germinal layer, or skin-sense layer. The underlying skin (corium or hypodermis) consists chiefly of connective tissue, contains numerous blood-vessels and nerves, and has a totally different origin. It comes from the outermost parietal stratum of the middle germinal layer, or the skin-fibre layer. The corium is much thicker than the epidermis. In its deeper strata (the subcutis) there are clusters of fat-cells (Fig. 284 h). Its uppermost stratum (the cutis proper, or the papillary stratum) forms, over almost the whole surface of the body, a number of conical microscopic papillæ (something like warts), which push into the overlying epidermis (c). These tactile or sensory particles contain the finest sensory organs of the skin, the touch corpuscles. Others contain merely end-loops of the blood-vessels that nourish the skin (c, d). The various parts of the corium arise by division of labour from the originally homogeneous cells of the cutis-plate, the outermost lamina of the mesodermic skin-fibre layer (Fig. 145 hpr, and Figs. 161, 162 cp).
In the same way, all the parts and appendages of the epidermis develop by differentiation from the homogeneous cells of this horny plate (Fig. 285). At an early stage the simple cellular layer of this horny plate divides into two. The inner and softer stratum (Fig. 284 b) is known as the mucous stratum, the outer and harder (a) as the horny (corneous) stratum. This horny layer is being constantly used up and rubbed away at the surface; new layers of cells grow up in their place out of the underlying mucous stratum. At first the epidermis is a simple covering of the surface of the body. Afterwards various appendages develop from it, some internally, others externally. The internal appendages are the cutaneous glands—sweat, fat, etc. The external appendages are the hairs and nails.
The cutaneous glands are originally merely solid cone-shaped growths of the epidermis, which sink into the underlying corium (Fig. 286 1). Afterwards a canal (2, 3) is formed inside them, either by the softening and dissolution of the central cells or by the secretion of fluid internally. Some of the glands, such as the sudoriferous, do not ramify (Fig. 284 efg). These glands, which secrete the perspiration, are very long, and have a spiral coil at the end, but they never ramify; so also the wax-glands of the ears. Most of the other cutaneous glands give out buds and ramify; thus, for instance, the lachrymal glands of the upper eye-lid that secrete tears (Fig. 286), and the sebaceous glands which secrete the fat in the skin and generally open into the hair-follicles. Sudoriferous and sebaceous glands are found only in mammals. But we find lachrymal glands in all the three classes of Amniotes—reptiles, birds, and mammals. They are wanting in the lower aquatic vertebrates.
Fig. 286—Rudimentary lachrymal glands from a human embryo of four months. (From Kölliker.) 1 earliest structure, in the shape of a simple solid cone, 2 and 3 more advanced structures, ramifying and hollowing out. a solid buds, e cellular coat of the hollow buds, f structure of the fibrous envelope, which afterwards forms the corium about the glands.
The mammary glands (Figs. 287, 288) are very remarkable; they are found in all mammals, and in these alone. They secrete the milk for the feeding of the new-born mammal. In spite of their unusual size these structures are nothing more than large sebaceous glands in the skin. The milk is formed by the liquefaction of the fatty milk-cells inside the branching mammary-gland tubes (Fig. 287 c), in the same way as the skin-grease or hair-fat, by the solution of fatty cells inside the sebaceous glands. The outlets of the mammary glands enlarge and form sac-like mammary ducts (b); these narrow again (a), and open in the teats or nipples of the breast by sixteen to twenty-four fine apertures. The first structure of this large and elaborate gland is a very simple cone in the epidermis, which penetrates into the corium and ramifies. In the new-born infant it consists of twelve to eighteen radiating lobes (Fig. 288). These gradually ramify, their ducts become hollow and larger, and rich masses of fat accumulate between the lobes. Thus is formed the prominent female breast (mamma), on the top of which rises the teat or nipple (mammilla). The latter is only developed later on, when the mammary gland is fully-formed; and this ontogenetic phenomenon is extremely interesting, because the earlier mammals (the stem-forms of the whole class) have no teats. In them the milk comes out through a flat portion of the ventral skin that is pierced like a sieve, as we still find in the lowest living mammals, the oviparous Monotremes of Australia. The young animal licks the milk from the mother instead of sucking it. In many of the lower mammals we find a number of milk-glands at different parts of the ventral surface. In the human female there is usually only one pair of glands, at the breast; and it is the same with the apes, bats, elephants, and several other mammals. Sometimes, however, we find two successive pairs of glands (or even more) in the human female. Some women have four or five pairs of breasts, like pigs and hedgehogs (Fig. 103). This polymastism points back to an older stem-form. We often find these accessory breasts in the male also (Fig. 103 D). Sometimes, moreover, the normal mammary glands are fully developed and can suckle in the male; but as a rule they are merely rudimentary organs without functions in the male. We have already (Chapter XI) dealt with this remarkable and interesting instance of atavism.
While the cutaneous glands are inner growths of the epidermis, the appendages which we call hairs and nails are external local growths in it. The nails (Ungues) which form important protective structures on the back of the most sensitive parts of our limbs, the tips of the fingers and toes, are horny growths of the epidermis, which we share with the apes. The lower mammals usually have claws instead of them; the ungulates, hoofs. The stem-form of the mammals certainly had claws; we find them in a rudimentary form even in the salamander. The horny claws are highly developed in most of the reptiles (Fig. 264), and the mammals have inherited them from the earliest representatives of this class, the stem-reptiles (Tocosauria). Like the hoofs (ungulæ) of the Ungulates, the nails of apes and men have been evolved from the claws of the older mammals. In the human embryo the first rudiment of the nails is found (between the horny and the mucous stratum of the epidermis) in the fourth month. But their edges do not penetrate through until the end of the sixth month.
Fig. 287—The female breast (mamma) in vertical section. c racemose glandular lobes, b enlarged milk-ducts, a narrower outlets, which open into the nipple. (From H. Meyer.)
The most interesting and important appendages of the epidermis are the hairs; on account of their peculiar composition and origin we must regard them as highly characteristic of the whole mammalian class. It is true that we also find hairs in many of the lower animals, such as insects and worms. But these hairs, like the hairs of plants, are thread-like appendages of the surface, and differ entirely from the hairs of the mammals in the details of their structure and development.
The embryology of the hairs is known in all its details, but there are two different views as to their phylogeny. On the older view the hairs of the mammals are equivalent or homologous to the feathers of the bird or the horny scales of the reptile. As we deduce all three classes of Amniotes from a common stem-group, we must assume that these Permian stem-reptiles had a complete scaly coat, inherited from their Carboniferous ancestors, the mailed amphibia (Stegocephala); the bony scales of their corium were covered with horny scales. In passing from aquatic to terrestrial life the horny scales were further developed, and the bony scales degenerated in most of the reptiles. As regards the bird’s feathers, it is certain that they are modifications of the horny scales of their reptilian ancestors. But it is otherwise with the hairs of the mammals. In their case the hypothesis has lately been advanced on the strength of very extensive research, especially by Friedrich Maurer, that they have been evolved from the cutaneous sense-organs of amphibian ancestors by modification of functions; the epidermic structure is very similar in both in its embryonic rudiments. This modern view, which had the support of the greatest expert on the vertebrates, Carl Gegenbaur, can be harmonised with the older theory to an extent, in the sense that both formations, scales and hairs, were very closely connected originally. Probably the conical budding of the skin-sense layer grew up under the protection of the horny scale, and became an organ of touch subsequently by the cornification of the hairs; many hairs are still sensory organs (tactile hairs on the muzzle and cheeks of many mammals: pubic hairs).
This middle position of the genetic connection of scales and hairs was advanced in my Systematic Phylogeny of the Vertebrates (p. 433). It is confirmed by the similar arrangement of the two cutaneous formations. As Maurer pointed out, the hairs, as well as the cutaneous sense-organs and the scales, are at first arranged in regular longitudinal series, and they afterwards break into alternate groups. In the embryo of a bear two inches long, which I owe to the kindness of Herr von Schmertzing (of Arva Varallia, Hungary), the back is covered with sixteen to twenty alternating longitudinal rows of scaly protuberances (Fig. 289). They are at the same time arranged in regular transverse rows, which converge at an acute angle from both sides towards the middle of the back. The tip of the scale-like wart is turned inwards. Between these larger hard scales (or groups of hairs) we find numbers of rudimentary smaller hairs.
The human embryo is, as a rule, entirely clothed with a thick coat of fine wool during the last three or four weeks of gestation. This embryonic woollen coat (Lanugo) generally disappears in part during the last weeks of fœtal life but in any case, as a rule, it is lost immediately after birth, and is replaced by the thinner coat of the permanent hair. These permanent hairs grow out of hair-follicles, which are formed from the root-sheaths of the disappearing wool-fibres. The embryonic wool-coat usually, in the case of the human embryo, covers the whole body, with the exception of the palms of the hands and soles of the feet. These parts are always bare, as in the case of apes and of most other mammals. Sometimes the wool-coat of the embryo has a striking effect, by its colour, on the later permanent hair-coat. Hence it happens occasionally, for instance, among our Indo-Germanic races, that children of blond parents seem—to the dismay of the latter—to be covered at birth with a dark brown or even a black woolly coat. Not until this has disappeared do we see the permanent blond hair which the child has inherited. Sometimes the darker coat remains for weeks, and even months, after birth. This remarkable woolly coat of the human embryo is a legacy from the apes, our ancient long-haired ancestors.
Fig. 288—Mammary gland of a new-born infant, a original central gland, b small and c large buds of same. (From Langer.)
It is not less noteworthy that many of the higher apes approach man in the thinness of the hair on various parts of the body. With most of the apes, especially the higher Catarrhines (or narrow-nosed apes), the face is mostly, or entirely, bare, or at least it has hair no longer or thicker than that of man. In their case, too, the back of the head is usually provided with a thicker growth of hair; this is lacking, however, in the case of the bald-headed chimpanzee (Anthropithecus calvus). The males of many species of apes have a considerable beard on the cheeks and chin; this sign of the masculine sex has been acquired by sexual selection. Many species of apes have a very thin covering of hair on the breast and the upper side of the limbs—much thinner than on the back or the under side of the limbs. On the other hand, we are often astonished to find tufts of hair on the shoulders, back, and extremities of members of our Indo-Germanic and of the Semitic races. Exceptional hair on the face, as on the whole body, is hereditary in certain families of hairy men. The quantity and the quality of the hair on head and chin are also conspicuously transmitted in families. These extraordinary variations in the total and partial hairy coat of the body, which are so noticeable, not only in comparing different races of men, but also in comparing different families of the same race, can only be explained on the assumption that in man the hairy coat is, on the whole, a rudimentary organ, a useless inheritance from the more thickly-coated apes. In this man resembles the elephant, rhinoceros, hippopotamus, whale, and other mammals of various orders, which have also, almost entirely or for the most part, lost their hairy coats by adaptation.
The particular process of adaptation by which man lost the growth of hair on most parts of his body, and retained or augmented it at some points, was most probably sexual selection. As Darwin luminously showed in his Descent of Man, sexual selection has been very active in this respect. As the male anthropoid apes chose the females with the least hair, and the females favoured the males with the finest growths on chin and head, the general coating of the body gradually degenerated, and the hair of the beard and head was more strongly developed. The growth of hair at other parts of the body (arm-pit, pubic region) was also probably due to sexual selection. Moreover, changes of climate, or habits, and other adaptations unknown to us, may have assisted the disappearance of the hairy coat.
The fact that our coat of hair is inherited directly from the anthropoid apes is proved in an interesting way, according to Darwin, by the direction of the rudimentary hairs on our arms, which cannot be explained in any other way. Both on the upper and the lower part of the arm they point towards the elbow. Here they meet at an obtuse angle. This curious arrangement is found only in the anthropoid apes—gorilla, chimpanzee, orang, and several species of gibbons—besides man (Figs. 203, 207). In other species of gibbon the hairs are pointed towards the hand both in the upper and lower arm, as in the rest of the mammals. We can easily explain this remarkable peculiarity of the anthropoids and man on the theory that our common ancestors were accustomed (as the anthropoid apes are to-day) to place their hands over their heads, or across a branch above their heads, during rain. In this position, the fact that the hairs point downwards helps the rain to run off. Thus the direction of the hair on the lower part of our arm reminds us to-day of that useful custom of our anthropoid ancestors.
The nervous system in man and all the other Vertebrates is, when fully formed, an extremely complex apparatus, that we may compare, in anatomic structure and physiological function, with an extensive telegraphic system. The chief station of the system is the central marrow or central nervous system, the innumerable ganglionic cells or neurona (Fig. 9)of which are connected by branching processes with each other and with numbers of very fine conducting wires. The latter are the peripheral and ubiquitous nerve-fibres; with their terminal apparatus, the sense-organs, etc., they constitute the conducting marrow or peripheral nervous system. Some of them—the sensory nerve-fibres—conduct the impressions from the skin and other sense-organs to the central marrow; others—the motor nerve-fibres—convey the commands of the will to the muscles.
The central nervous system or central marrow (medulla centralis) is the real organ of psychic action in the narrower sense. However we conceive the intimate connection of this organ and its functions, it is certain that its characteristic actions, which we call sensation, will, and thought, are inseparably dependent on the normal development of the material organ in man and all the higher animals. We must, therefore, pay particular attention to the evolution of the latter. As it can give us most important information regarding the nature of the “soul,” it should be full of interest. If the central marrow develops in just the same way in the human embryo as in the embryo of the other mammals, the evolution of the human psychic organ from the central organ of the other mammals, and through them from the lower vertebrates, must be beyond question. No one can doubt the momentous bearing of these embryonic phenomena.
Fig. 290—Human embryo, three months old,
from the dorsal side: brain and spinal cord exposed. (From Kölliker.)
h cerebral hemispheres (fore brain), m corpora quadrigemina
(middle brain), c cerebellum (hind brain): under the latter is the
triangular medulla oblongata (after brain).
Fig. 291—Central
marrow of a human embryo, four months old, from the back. (From
Kölliker.) h large hemispheres, v quadrigemina, c
cerebellum, mo medulla oblongata: underneath it the spinal
cord.
In order to understand them fully we must first say a word or two of the general form and the anatomic composition of the mature human central marrow. Like the central nervous system of all the other Craniotes, it consists of two parts, the head-marrow or brain (medulla capitis or encephalon) and the spinal-marrow (medulla spinalis or notomyelon). The one is enclosed in the bony skull, the other in the bony vertebral column. Twelve pairs of cerebral nerves proceed from the brain, and thirty-one pairs of spinal nerves from the spinal cord, to the rest of the body (Fig. 171). On general anatomic investigation the spinal marrow is found to be a cylindrical cord, with a spindle-shaped bulb both in the region of the neck above (at the last cervical vertebra) and the region of the loins (at the first lumbar vertebra) below (Fig. 291). At the cervical bulb the strong nerves of the upper limbs, and at the lumbar bulb those of the lower limbs, proceed from the spinal cord. Above, the latter passes into the brain through the medulla oblongata (Fig. 291 mo). The spinal cord seems to be a thick mass of nervous matter, but it has a narrow canal at its axis, which passes into the further cerebral ventricles above, and is filled, like these, with a clear fluid.
The brain is a large nerve-mass, occupying the greater part of the skull, of most elaborate structure. On general examination it divides into two parts, the cerebrum and cerebellum. The cerebrum lies in front and above, and has the familiar characteristic convolutions and furrows on its surface (Figs. 292, 293). On the upper side it is divided by a deep longitudinal fissure into two halves, the cerebral hemispheres; these are connected by the corpus callosum. The large cerebrum is separated from the small cerebellum by a deep transverse furrow. The latter lies behind and below, and has also numbers of furrows, but much finer and more regular, with convolutions between, at its surface. The cerebellum also is divided by a longitudinal fissure into two halves, the “small hemispheres”; these are connected by a worm-shaped piece, the vermis cerebelli, above, and by the broad pons Varolii below (Fig. 292 VI).
Fig. 292—The human brain, seen from below. (From H. Meyer.) Above (in front) is the cerebrum with its extensive branching furrows; below (behind) the cerebellum with its narrow parallel furrows. The Roman numbers indicate the roots of the twelve pairs of cerebral nerves in a series towards the rear.
But comparative anatomy and ontogeny teach us that in man and all the other Craniotes the brain is at first composed, not of these two, but of three, and afterwards five, consecutive parts. These are found in just the same form—as five consecutive vesicles—in the embryo of all the Craniotes, from the Cyclostoma and fishes to man. But, however much they agree in their rudimentary condition, they differ considerably afterwards. In man and the higher mammals the first of these ventricles, the cerebrum, grows so much that in its mature condition it is by far the largest and heaviest part of the brain. To it belong not only the large hemispheres, but also the corpus callosum that unites them, the olfactory lobes, from which the olfactory nerves start, and most of the structures that are found at the roof and bottom of the large lateral ventricles inside the two hemispheres, such as the corpora striata. On the other hand, the optic thalami, which lie between the latter, belong to the second division, which develops from the “intermediate brain ”; to the same section belong the single third cerebral ventricle and the structures that are known as the corpora geniculata, the infundibulum, and the pineal gland. Behind these parts we find, between the cerebrum and cerebellum, a small ganglion composed of two prominences, which is called the corpus quadrigeminum on account of a superficial transverse fissure cutting across (Figs. 290 m and 291 v). Although this quadrigeminum is very insignificant in man and the higher mammals, it forms a special third section, greatly developed in the lower vertebrates, the “middle brain.” The fourth section is the “hind-brain” or little brain (cerebellum) in the narrower sense, with the single median part, the vermis, and the pair of lateral parts, the “small hemispheres” (Fig. 291 c). Finally, we have the fifth and last section, the medulla oblongata (Fig. 291 mo), which contains the single fourth cerebral cavity and the contiguous parts (pyramids, olivary bodies, corpora restiformia). The medulla oblongata passes straight into the medulla spinalis (spinal cord). The narrow central canal of the spinal cord continues above into the quadrangular fourth cerebral cavity of the medulla oblongata, the floor of which is the quadrangular depression. From here a narrow duct, called “the aqueduct of Sylvius,” passes through the corpus quadrigeminum to the third cerebral ventricle, which lies between the two optic thalami; and this in turn is connected with the pairs of lateral ventricles which lie to the right and left in the large hemispheres. Thus all the cavities of the central marrow are directly interconnected. All these parts of the brain have an infinitely complex structure in detail, but we cannot go into this. Although it is much more elaborate in man and the higher Vertebrates than in the lower classes, it develops in them all from the same rudimentary structure, the five simple cerebral vesicles of the embryonic brain.
But before we consider the development of the complicated structure of the brain from this simple series of vesicles, let us glance for a moment at the lower animals, which have no brain. Even in the skull-less vertebrate, the Amphioxus, we find no independent brain, as we have seen. The whole central marrow is merely a simple cylindrical cord which runs the length of the body, and ends equally simply at both extremities—a plain medullary tube. All that we can discover is a small vesicular bulb at the foremost part of the tube, a degenerate rudiment of a primitive brain. We meet the same simple medullary tube in the first structure of the ascidia larva, in the same characteristic position, above the chorda. On closer examination we find here also a small vesicular swelling at the fore end of the tube, the first trace of a differentiation of it into brain and spinal cord. It is probable that this differentiation was more advanced in the extinct Provertebrates, and the brain-bulb more pronounced (Figs. 98–102). The brain is phylogenetically older than the spinal cord, as the trunk was not developed until after the head. If we consider the undeniable affinity of the Ascidiæ to the Vermalia, and remember that we can trace all the Chordonia to lower Vermalia, it seems probable that the simple central marrow of the former is equivalent to the simple nervous ganglion, which lies above the gullet in the lower worms, and has long been known as the “upper pharyngeal ganglion” (ganglion pharyngeum superius); it would be better to call it the primitive or vertical brain (acroganglion).
Probably this upper pharyngeal ganglion of the lower worms is the structure from which the complex central marrow of the higher animals has been evolved. The medullary tube of the Chordonia has been formed by the lengthening of the vertical brain on the dorsal side. In all the other animals the central nervous system has been developed in a totally different way from the upper pharyngeal ganglion; in the Articulates, especially, a pharyngeal ring, with ventral marrow, has been added. The Molluscs also have a pharyngeal ring, but it is not found in the Vertebrates. In these the central marrow has been prolonged down the dorsal side; in the Articulates down the ventral side. This fact proves of itself that there is no direct relationship between the Vertebrates and the Articulates. The unfortunate attempts to derive the dorsal marrow of the former from the ventral marrow of the latter have totally failed (cf. p. 219).
Fig. 293—The human brain, seen from the left. (From H. Meyer.) The furrows of the cerebrum are indicated by thick, and those of the cerebellum by finer lines. Under the latter we can see the medulla oblongata. f1–f2 frontal convolutions, C central convolutions, S fissure of Sylvius, T temporal furrow, Pa parietal lobes, An angular gyrus, Po parieto-occipital fissure.
When we examine the embryology of the human nervous system, we must start from the important fact, which we have already seen, that the first structure of it in man and all the higher Vertebrates is the simple medullary tube, and that this separates from the outer germinal layer in the middle line of the sole-shaped embryonic shield. As the reader will remember, the straight medullary furrow first appears in the middle of the sandal-shaped embryonic shield. At each side of it the parallel borders curve over in the form of dorsal or medullary swellings. These bend together with their free borders, and thus form the closed medullary tube (Figs. 133–137). At first this tube lies directly underneath the horny plate; but it afterwards travels inwards, the upper edges of the provertebral plates growing together between the horny plate and the tube, joining above the latter, and forming a completely closed canal. As Gegenbaur very properly observes, “this gradual imbedding in the inner part of the body is a process acquired with the progressive differentiation and the higher potentiality that this secures; by this process the organ of greater value to the organism is buried within the frame.” (Cf. Figs. 143–146).
In the Cyclostoma—a stage above the Acrania—the fore end of the cylindrical medullary tube begins early to expand into a pear-shaped vesicle; this is the first outline of an independent brain. In this way the central marrow of the Vertebrates divides clearly into its two chief sections, brain and spinal cord. The simple vesicular form of the brain, which persists for some time in the Cyclostoma, is found also at first in all the higher Vertebrates (Fig. 153 hb). But in these it soon passes away, the one vesicle being divided into several successive parts by transverse constrictions. There are first two of these constrictions, dividing the brain into three consecutive vesicles (fore brain, middle brain, and hind brain, Fig. 154 v, m, h). Then the first and third are sub-divided by fresh constrictions, and thus we get five successive sections (Fig. 155).
Fig. 294–296—Central marrow of the human embryo from the seventh week, 4/5 inch long. (From Kölliker.) Fig. 294. The brain from above, v fore brain, z intermediate brain, m middle brain, h hind brain, n after brain. Fig. 2955. The brain with the uppermost part of the cord, from the left. Fig. 296. Back view of the whole embryo: brain and spinal cord exposed.
In all the Craniotes, from the Cyclostoma up to man, the same parts develop from these five original cerebral vesicles, though in very different ways. The first vesicle, the fore brain (Fig. 155 v), forms by far the largest part of the cerebrum—namely, the large hemispheres, the olfactory lobes, the corpora striata, the callosum, and the fornix. From the second vesicle, the intermediate brain (z), originate especially the optic thalami, the other parts that surround the third cerebral ventricle, and the infundibulum and pineal gland. The third vesicle, the middle brain (m), produces the corpora quadrigemina and the aqueduct of Sylvius. From the fourth vesicle, the hind brain (h), develops the greater part of the cerebellum—namely, the vermis and the two small hemispheres. Finally, the fifth vesicle, the after brain (n), forms the medulla oblongata, with the quadrangular pit (the floor of the fourth ventricle), the pyramids, olivary bodies, etc.
We must certainly regard it as a comparative-anatomical and ontogenetic fact of the greatest significance that in all the Craniotes, from the lowest Cyclostomes and fishes up to the apes and man, the brain develops in just the same way in the embryo. The first rudiment of it is always a simple vesicular enlargement of the fore end of the medullary tube. In every case, first three, then five, vesicles develop from this bulb, and the permanent brain with all its complex anatomic structures, of so great a variety in the various classes of Vertebrates, is formed from the five primitive vesicles. When we compare the mature brain of a fish, an amphibian, a reptile, a bird, and a mammal, it seems incredible that we can trace the various parts of these organs, that differ so much internally and externally, to common types. Yet all these different Craniote brains have started with the same rudimentary structure. To convince ourselves of this we have only to compare the corresponding stages of development of the embryos of these different animals.
This comparison is extremely instructive. If we extend it through the whole series of the Craniotes, we soon discover this interesting fact: In the Cyclostomes (the Myxinoida and Petromyzonta), which we have recognised as the lowest and earliest Craniotes, the whole brain remains throughout life at a very low stage, which is very brief and passing in the embryos of the higher Craniotes; they retain the five original sections of the brain unchanged. In the fishes we find an essential and considerable modification of the five vesicles; it is clearly the brain of the Selachii in the first place, and subsequently the brain of the Ganoids, from which the brain of the rest of the fishes on the one hand and of the Dipneusts and Amphibia, and through these of the higher Vertebrates, on the other hand, must be derived. In the fishes and Amphibia (Fig. 300) there is a preponderant development of the middle brain, and also the after brain, the first, second, and fourth sections remaining very primitive. It is just the reverse in the higher Vertebrates, in which the first and third sections, the cerebrum and cerebellum, are exceptionally developed; while the middle brain and after brain remain small. The corpora quadrigemina are mostly covered by the cerebrum, and the oblongata by the cerebellum. But we find a number of stages of development within the higher Vertebrates themselves. From the Amphibia upwards the brain (and with it the psychic life) develops in two different directions; one of these is followed by the reptiles and birds, and the other by the mammals. The development of the first section, the fore brain, is particularly characteristic of the mammals. It is only in them that the cerebrum becomes so large as to cover all the other parts of the brain (Figs. 293, 301–304).
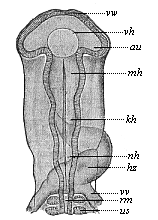
Fig. 297—Head of a chick embryo (hatched fifty-eight hours), from the back. (From Mihalkovics.) vw anterior wall of the fore brain. vh its ventricle. au optic vesicles, mh middle brain, kh hind brain, nh after brain, hz heart (seen from below), vw vitelline veins, us primitive segment, rm spinal cord.
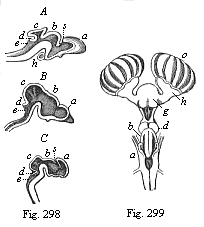
Fig. 298—Brain of three craniote embryos in
vertical section. A of a shark (Heptarchus), B of a
serpent (Coluber), C of a goat (Capra). a fore
brain, b intermediate brain, c middle brain, d hind brain,
e after brain, s primitive cleft. (From Gegenbaur.)
Fig. 299—Brain of a shark (Scyllium), back view. g
fore-brain, h olfactory lobes, which send the large olfactory nerves to
the nasal capsule (o), d intermediate brain, b middle
brain; behind this the insignificant structure of the hind brain, a
after brain. (From Gegenbaur.)
There are also notable variations in the relative position of the cerebral vesicles. In the lower Craniotes they lie originally almost in the same plane. When we examine the brain laterally, we can cut through all five vesicles with a straight line. But in the Amniotes there is a considerable curve in the brain along with the bending of the head and neck; the whole of the upper dorsal surface of the brain develops much more than the under ventral surface. This causes a curve, so that the parts come to lie as follows: The fore brain is right in front and below, the intermediate brain a little higher, and the middle brain highest of all; the hind brain lies a little lower, and the after brain lower still. We find this only in the Amniotes—the reptiles, birds, and mammals.
Thus, while the brain of the mammals agrees a good deal in general growth with that of the birds and reptiles, there are some striking differences between the two. In the Sauropsids (birds and reptiles) the middle brain and the middle part of the hind brain are well developed. In the mammals these parts do not grow, and the fore-brain develops so much that it overlies the other vesicles. As it continues to grow towards the rear, it at last covers the whole of the rest of the brain, and also encloses the middle parts from the sides (Figs. 301–303). This process is of great importance, because the fore brain is the organ of the higher psychic life, and in it those functions of the nerve-cells are discharged which we sum up in the word “soul.” The highest achievements of the animal body—the wonderful manifestations of consciousness and the complex molecular processes of thought—have their seat in the fore brain. We can remove the large hemispheres, piece by piece, from the mammal without killing it, and we then see how the higher functions of consciousness, thought, will, and sensation, are gradually destroyed, and in the end completely extinguished. If the animal is fed artificially, it may be kept alive for a long time, as the destruction of the psychic organs by no means involves the extinction of the faculties of digestion, respiration, circulation, urination—in a word, the vegetative functions. It is only conscious sensation, voluntary movement, thought, and the combination of various higher psychic functions that are affected.
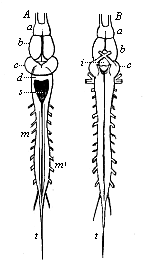
Fig. 300—Brain and spinal cord of the frog. A from the dorsal, B from the ventral side. a olfactory lobes before the (b) fore brain, i infundibulum at the base of the intermediate brain, c middle brain, d hind brain, s quadrangular pit in the after brain, m spinal cord (very short in the frog), m′ roots of the spinal nerves, t terminal fibres of the spinal cord. (From Gegenbaur.)
The fore brain, the organ of these functions, only attains this high level of development in the more advanced Placentals, and thus we have the simple explanation of the intellectual superiority of the higher mammals. The soul of most of the lower Placentals is not much above that of the reptiles, but among the higher Placentals we find an uninterrupted gradation of mental power up to the apes and man. In harmony with this we find an astonishing variation in the degree of development of their fore brain, not only qualitatively, but also quantitatively. The mass and weight of the brain are much greater in modern mammals, and the differentiation of its various parts more important, than in their extinct Tertiary ancestors. This can be shown paleontologically in any particular order. The brains of the living ungulates are (relatively to the size of the body) four to six times (in the highest groups even eight times) as large as those of their earlier Tertiary ancestors, the well-preserved skulls of which enable us to determine the size and weight of the brain.
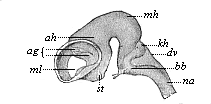
Fig. 301—Brain of an ox-embryo, two inches in length. (From Mihalkovics.) Left view; the lateral wall of the left hemisphere has been removed, st corpora striata, ml Monro-foramen, ag arterial plexus, ah Ammon’s horn, mh middle brain, kh cerebellum, dv roof of the fourth ventricle, bb pons Varolii, na medulla oblongata.
Fig. 302—Brain of a human embryo, twelve weeks old. (From Mihalkovics.) Seen from behind and above. ms mantle-furrow, mh corpora quadrigemina (middle brain), vs anterior medullary ala, kh cerebellum, vv fourth ventricle, na medulla oblongata.
In the lower mammals the surface of the cerebral hemispheres is quite smooth and level, as in the rabbit (Fig. 304). Moreover, the fore brain remains so small that it does not cover the middle brain. At a stage higher the middle brain is covered, but the hind brain remains free. Finally, in the apes and man, the latter also is covered by the fore brain. We can trace a similar gradual development in the fissures and convolutions that are found on the surface of the cerebrum of the higher mammals (Figs. 292, 293). If we compare different groups of mammals in regard to these fissures and convolutions, we find that their development proceeds step by step with the advance of mental life.
Fig. 303—Brain of a human embryo, twenty-four weeks old, halved in the median plane: right hemisphere seen from inside. (From Mihalkovics.) rn olfactory nerve, tr funnel of the intermediate brain, vc anterior commissure, ml Monro-foramen, gw fornix, ds transparent sheath, bl corpus callosum, br fissure at its border, hs occipital fissure, zh cuneus, sf occipital transverse fissure, zb pineal gland, mh corpora quadrigemina, kh cerebellum.
Of late years great attention has been paid to this special branch of cerebral anatomy, and very striking individual differences have been detected within the limits of the human race. In all human beings of special gifts and high intelligence the convolutions and fissures are much more developed than in the average man; and they are more developed in the latter than in idiots and others of low mental capacity. There is a similar gradation among the mammals in the internal structure of the fore brain. In particular the corpus callosum, that unites the two cerebral hemispheres, is only developed in the Placentals. Other structures—for instance, in the lateral ventricles—that seem at first to be peculiar to man, are also found in the higher apes, and these alone. It was long thought that man had certain distinctive organs in his cerebrum which were not found in any other animal. But careful examination has discovered that this is not the case, but that the characteristic features of the human brain are found in a rudimentary form in the lower apes, and are more or less fully developed in the higher apes. Huxley has convincingly shown, in his Man’s Place in Nature (1863), that the differences in the formation of the brain within the ape-group constitute a deeper gulf between the lower and higher apes than between the higher apes and man.
Fig. 304—Brain of the rabbit. A from the dorsal, B from the ventral side, lo olfactory lobes, I fore brain, h hypophysis at the base of the intermediate brain, III middle brain, IV hind brain, V after brain, 2 optic nerve, 3 oculo-motor nerve, 5–8 cerebral nerves. In A the roof of the right hemisphere (I) is removed, so that we can see the corpora striata in the lateral ventricle. (From Gegenbaur.)
The comparative anatomy and physiology of the brain of the higher and lower mammals are very instructive, and give important information in connection with the chief questions of psychology.
The central marrow (brain and spinal cord) develops from the medullary tube in man just as in all the other mammals, and the same applies to the conducting marrow or “peripheral nervous system.” It consists of the sensory nerves, which conduct centripetally the impressions from the skin and the sense-organs to the central marrow, and of the motor nerves, which convey centrifugally the movements of the will from the central marrow to the muscles. All these peripheral nerves grow out of the medullary tube (Fig. 171), and are, like it, products of the skin-sense layer.
The complete agreement in the structure and development of the psychic organs which we find between man and the highest mammals, and which can only be explained by their common origin, is of profound importance in the monistic psychology. This is only seen in its full light when we compare these morphological facts with the corresponding physiological phenomena, and remember that every psychic action requires the complete and normal condition of the correlative brain structure for its full and normal exercise. The very complex molecular movements inside the neural cells, which we describe comprehensively as “the life of the soul,” can no more exist in the vertebrate, and therefore in man, without their organs than the circulation without the heart and blood. And as the central marrow develops in man from the same medullary tube as that of the other vertebrates, and as man shares the characteristic structure of his cerebrum (the organ of thought) with the anthropoid apes, his psychic life also must have the same origin as theirs.
If we appreciate the full weight of these morphological and physiological facts, and put a proper phylogenetic interpretation on the observations of embryology, we see that the older idea of the personal immortality of the human soul is scientifically untenable. Death puts an end, in man as in any other vertebrate, to the physiological function of the cerebral neurona, the countless microscopic ganglionic cells, the collective activity of which is known as “the soul.” I have shown this fully in the eleventh chapter of my Riddle of the Universe.
The sense-organs are indubitably among the most important and interesting parts of the human body; they are the organs by means of which we obtain our knowledge of objects in the surrounding world. Nihil est in intellectu quod non prius fuerit in sensu. They are the first sources of the life of the soul. There is no other part of the body in which we discover such elaborate anatomical structures, co-operating with a definite purpose; and there is no other organ in which the wonderful and purposive structure seems so clearly to compel us to admit a Creator and a preconceived plan. Hence we find special efforts made by dualists to draw our attention here to the “wisdom of the Creator” and the design visible in his works. As a matter of fact, you will discover, on mature reflection, that on this theory the Creator is at bottom only playing the part of a clever mechanic or watch-maker; all these familiar teleological ideas of Creator and creation are based, in the long run, on a similar childlike anthropomorphism.
However, we must grant that at the first glance the teleological theory seems to give the simplest and most satisfactory explanation of these purposive structures. If we merely examine the structure and functions of the most advanced sense-organs, it seems impossible to explain them without postulating a creative act. Yet evolution shows us quite clearly that this popular idea is totally wrong. With its assistance we discover that the purposive and remarkable sense-organs were developed, like all other organs, without any preconceived design—developed by the same mechanical process of natural selection, the same constant correlation of adaptation and heredity, by which the other purposive structures in the animal frame were slowly and gradually brought forth in the struggle for life.
Like most other Vertebrates, man has six sensory organs, which serve for eight different classes of sensations. The skin serves for sensations of pressure and temperature. This is the oldest, lowest, and vaguest of the sense-organs; it is distributed over the surface of the body. The other sensory activities are localised. The sexual sense is bound up with the skin of the external sexual organs, the sense of taste with the mucous lining of the mouth (tongue and palate), and the sense of smell with the mucous lining of the nasal cavity. For the two most advanced and most highly differentiated sensory functions there are special and very elaborate mechanical structures—the eye for the sense of sight, and the ear for the sense of hearing and space (equilibrium).
Comparative anatomy and physiology teach us that there are no differentiated sense-organs in the lower animals; all their sensations are received by the surface of the skin. The undifferentiated skin-layer or ectoderm of the Gastræa is the simple stratum of cells from which the differentiated sense-organs of all the Metazoa (including the Vertebrates) have been evolved. Starting from the assumption that necessarily only the superficial parts of the body, which are in direct touch with the outer world, could be concerned in the origin of sensations, we can see at once that the sense-organs also must have arisen there. This is really the case. The chief part of all the sense-organs originates from the skin-sense layer, partly directly from the horny plate, partly from the brain, the foremost part, of the medullary tube, after it has separated from the horny plate. If we compare the embryonic development of the various sense-organs, we see that they all make their appearance in the simplest conceivable form; the wonderful contrivances that make the higher sense-organs among the most remarkable and elaborate structures in the body develop only gradually. In the phylogenetic explanation of them comparative anatomy and ontogeny achieve their greatest triumphs. But at first all the sense-organs are merely parts of the skin in which sensory nerves expand. These nerves themselves were originally of a homogeneous character. The different functions or specific energies of the differentiated sense-nerves were only gradually developed by division of labour. At the same time, their simple terminal expansions in the skin were converted into extremely complex organs.
The great instructiveness of these historical facts in connection with the life of the soul is not difficult to see. The whole philosophy of the future will be transformed as soon as psychology takes cognisance of these genetic phenomena and makes them the basis of its speculations. When we examine impartially the manuals of psychology that have been published by the most distinguished speculative philosophers and are still widely distributed, we are astonished at the naivete with which the authors raise their airy metaphysical speculations, regardless of the momentous embryological facts that completely refute them. Yet the science of evolution, in conjunction with the great advance of the comparative anatomy and physiology of the sense-organs, provides the one sound empirical basis of a natural psychology.
Fig. 305—Head of a shark (Scyllium), from the ventral side. m mouth, o olfactory pits, r nasal groove, n nasal fold in natural position, n′ nasal fold drawn up. (The dots are openings of the mucous canals.) (From Gegenbaur.)
In respect of the terminal expansions of the sensory nerves, we can distribute the human sense-organs in three groups, which correspond to three stages of development. The first group comprises those organs the nerves of which spread out quite simply in the free surface of the skin itself (organs of the sense of pressure, warmth, and sex). In the second group the nerves spread out in the mucous coat of cavities which are at first depressions in or invaginations of the skin (organs of the sense of smell and taste). The third group is formed of the very elaborate organs, the nerves of which spread out in an internal vesicle, separated from the skin (organs of the sense of sight, hearing, and space).
There is little to be said of the development of the lower sense-organs. We have already considered (p. 268) the organ of touch and temperature in the skin. I need only add that in the corium of man and all the higher Vertebrates countless microscopic sense-organs develop, but the precise relation of these to the sensations of pressure or resistance, of warmth and cold, has not yet been explained. Organs of this kind, in or on which sensory cutaneous nerves terminate, are the “tactile corpuscles” (or the Pacinian corpuscles) and end-bulbs. We find similar corpuscles in the organs of the sexual sense, the male penis and the female clitoris; they are processes of the skin, the development of which we will consider later (together with the rest of the sexual parts, Chapter XXIX). The evolution of the organ of taste, the tongue and palate, will also be treated later, together with that of the alimentary canal to which these parts belong (Chapter XXVII). I will only point out for the present that the mucous coat of the tongue and palate, in which the gustatory nerve ends, originates from a part of the outer skin. As we have seen, the whole of the mouth-cavity is formed, not as a part of the gut-tube proper, but as a pit-like fold in the outer skin (p. 139). Its mucous lining is therefore formed, not from the visceral, but from the cutaneous layer, and the taste-cells at the surface of the tongue and palate are not products of the gut-fibre layer, but of the skin-sense layer.
Fig. 306 and 307—Head of a chick embryo, three days old: 2.306 front view, 2.307 from the right. n rudimentary nose (olfactory pits), l rudimentary eyes (optic pits), g rudimentary ear (auscultory pit), v fore brain, gl eye-cleft, o process of upper jaw, u process of lower jaw of the first gill-arch.
Fig. 308—Head of a chick embryo, four days old, from below.
n nasal pit, o upper-jaw process of the first gill-arch, u
lower-jaw process of same, k″ second gill-arch, sp choroid
fissure of eye, s gullet.
Fig. 309 and 310—Heads of chick embryos: 309 from the end of the
fourth, 310 from the beginning of the fifth week. Letters as in Fig. 308,
except: in inner, an outer, nasal process, nf nasal furrow, st
frontal process, m mouth. (From Kölliker.).
This applies also to the mucous lining of the olfactory organ, the nose. However, the development of this organ is much more interesting. Although the nose seems superficially to be simple and single, it really consists, in man and all other Gnathostomes, of two completely separated halves, the right and left cavities. They are divided by a vertical partition, so that the right nostril leads into the right cavity alone and the left nostril into the left cavity. They open internally (and separately) by the posterior nasal apertures into the pharynx, so that we can get direct into the gullet through the nasal passages without touching the mouth. This is the way the air usually passes in respiration; the mouth being closed, it goes through the nose into the gullet, and through the larynx and bronchial tubes into the lungs. The nasal cavities are separated from the mouth by the horizontal bony palate, to which is attached behind (as a dependent process) the soft palate with the uvula. In the upper and hinder parts of the nasal cavities the olfactory nerve, the first pair of cerebral nerves, expands in the mucous coat which clothes them. The terminal branches of it spread partly over the septum (partition), partly on the side walls of the internal cavities, to which are attached the turbinated bones. These bones are much more developed in many of the higher mammals than in man, but there are three of them in all mammals. The sensation of smell arises by the passage of a current of air containing odorous matter over the mucous lining of the cavities, and stimulating the olfactory cells of the nerve-endings.
Man has all the features which distinguish the olfactory organ of the mammals from that of the lower Vertebrates. In all essential points the human nose entirely resembles that of the Catarrhine apes, some of which have quite a human external nose (compare the face of the long-nosed apes). However, the first structure of the olfactory organ in the human embryo gives no indication of the future ample proportions of our catarrhine nose. It has the form in which we find it permanently in the fishes—a couple of simple depressions in the skin at the outer surface of the head. We find these blind olfactory pits in all the fishes; sometimes they lie near the eyes, sometimes more forward at the point of the muzzle, sometimes lower down, near the mouth (Fig. 249).
Fig. 311—Frontal section of the mouth and throat of a human embryo, neck half-inch long. “Invented” by Wilhelm His. The vertical section (in the frontal plane, from left to right) is so constructed that we see the nasal pits in the upper third of the figure and the eyes at the sides: in the middle third the primitive gullet with the gill-clefts (gill-arches in section); in the lower third the pectoral cavity with the bronchial tubes and the rudimentary lungs.
This first rudimentary structure of the double nose is the same in all the Gnathostomes; it has no connection with the primitive mouth. But even in a section of the fishes a connection of this kind begins to make its appearance, a furrow in the surface of the skin running from each side of the nasal pit to the nearest corner of the mouth. This furrow, the nasal groove or furrow (Fig. 305 r), is very important. In many of the sharks, such as the Scyllium, a special process of the frontal skin, the nasal fold or internal nasal process, is formed internally over the groove (n, n″). In contrast to this the outer edge of the furrow rises in an “external nasal process.” As the two processes meet and coalesce over the nasal groove in the Dipneusts and Amphibia, it is converted into a canal, the nasal canal. Henceforth we can penetrate from the external pits through the nasal canals direct into the mouth, which has been formed quite independently. In the Dipneusts and the lower Amphibia the internal aperture of the nasal canals lies in front (behind the lips); in the higher Amphibia it is right behind. Finally, in the three higher classes of Vertebrates the primary mouth-cavity is divided by the formation of the horizontal palate-roof into two distinct cavities—the upper (secondary) nasal cavity and the lower (secondary) mouth-cavity. The nasal cavity in turn is divided by the construction of the vertical septum into two halves—right and left.
Fig. 312—Diagrammatic section of the mouth-nose cavity. While the palate-plates (p) divide the original mouth-cavity into the lower secondary mouth (m) and the upper nasal cavity, the latter in turn is divided by the vertical partition (e) into two halves (n, n). (From Gegenbaur.)
Comparative anatomy shows us to-day, in the series of the double-nosed Vertebrates, from the fishes up to man, all the different stages in the development of the nose, which the advanced olfactory organ of the higher mammals has passed through at various periods in the course of its phylogeny. It first appears in the embryo of man and the higher Vertebrates, in which the double fish-nose persists throughout life. At an early stage, before there is any trace of the characteristic human face, a pair of small pits are formed in the head over the original mouth-cavity; these were first discovered by Baer, and rightly called the “olfactory pits” (Figs. 306 n, 307 n). These primitive nasal pits are quite separate from the rudimentary mouth, which also originates as a pit-like depression in the skin, in front of the blind fore end of the gut. Both the pair of nasal pits and the single mouth-pit (Fig. 310 m) are clothed with the horny plate. The original separation of the former from the latter is, however, presently abolished, a process forming above the mouth-pit—the “frontal process” (Fig. 309 st). Its outer edge rises to the right and left in the shape of two lateral processes; these are the inner nasal processes or folds (in). Opposite to these a parallel ridge is formed on either side between the eye and the nasal pit; these are the outer nasal processes (an). Thus between the inner and outer nasal processes a groove-like depression is formed on either side, which leads from the nasal pit towards the mouth-pit (m); this groove is, as the reader will guess, the same nasal furrow or groove that we have already seen in the shark (Fig. 305 r). As the parallel edges of the inner and outer nasal processes bend towards each other and join above the nasal groove, this is converted into a tube, the primitive nasal canal. Hence the nose of man and all the other Amniotes consists at this embryonic stage of a couple of narrow tubes, the nasal canals, which lead from the outer surface of the forehead into the rudimentary mouth. This transitory condition resembles that in which we find the nose permanently in the Dipneusts and Amphibia.
A cone-shaped structure, which grows from below towards the lower ends of the two nasal processes and joins with them, plays an important part in the conversion of the open nasal groove into the closed canal. This is the upper-jaw process (Figs. 306–310 o). Below the mouth-pit are the gill-arches, which are separated by the gill-clefts. The first of these gill-arches, and the most important for our purpose, which we may call the maxillary (jaw) arch, forms the skeleton of the jaws. Above at the basis a small process grows out of this first gill-arch; this is the upper-jaw process. The first gill-arch itself develops a cartilage at one of its inner sides, the “Meckel cartilage” (named after its discoverer), on the outer surface of which the lower jaw is formed (Figs. 306–310 u). The upper-jaw process forms the chief part of the skeleton of that jaw, the palate bone, and the pterygoid bone. On its outer side is afterwards formed the upper-jaw bone, in the narrower sense, while the middle part of the skeleton of the upper jaw, the intermaxillary, develops from the foremost part of the frontal process.
The two upper-jaw processes are of great importance in the further development of the face. From them is formed, growing into the primitive mouth-cavity, the important horizontal partition (the palate) that divides the former into two distinct cavities. The upper cavity, into which the nasal canals open, now develops into the nasal cavity, the air-passage and the organ of smell. The lower cavity forms the permanent secondary mouth (Fig. 312 m), the food-passage and the organ of taste. Both the upper and lower cavities open behind into the gullet (pharynx). The hard palate that separates them is formed by the joining of two lateral halves, the horizontal plates of the two upper-jaw processes, or the palate-plates (p). When these do not, sometimes, completely join in the middle, a longitudinal cleft remains, through which we can penetrate from the mouth straight into the nasal cavity. This is the malformation known as “wolf’s throat.” “Hare-lip” is the lesser form of the same defect. At the same time as the horizontal partition of the hard palate a vertical partition is formed by which the single nasal cavity is divided into two sections—a right and left half (Fig. 312 n, n).
Figs. 313 and 314—Upper part of the body of a human embryo, two-thirds of an inch long, of the sixth week; Fig. 313 from the left, Fig. 314 from the front. The origin of the nose and the upper lip from two lateral and originally separate halves can be clearly seen. Nose and upper lip are large in proportion to the rest of the face, and especially to the lower lip. (From Kollmann.)
The double nose has now acquired the characteristic form that man shares with the other mammals. Its further development is easy to follow; it consists of the formation of the inner and outer processes of the walls of the two cavities. The external nose is not formed until long after all these essential parts of the internal organ of smell. The first traces of it in the human embryo are found about the middle of the second month (Figs. 313–316). As can be seen in any human embryo during the first month, there is at first no trace of the external nose. It only develops afterwards from the foremost nasal part of the primitive skull, growing forwards from behind. The characteristic human nose is formed very late. Much stress is at times laid on this organ as an exclusive privilege of man. But there are apes that have similar noses, such as the long-nosed ape.
The evolution of the eye is not less interesting and instructive than that of the nose. Although this noblest of the sensory organs is one of the most elaborate and purposive on account of its optic perfection and remarkable structure, it nevertheless develops, without preconceived design, from a simple process of the outer germinal layer. The fully-formed human eye is a round capsule, the eye-ball (Fig. 317). This lies in the bony cavity of the skull, surrounded by protective fat and motor muscles. The greater part of it is taken up with a semi-fluid, transparent gelatinous substance, the corpus vitreum. The crystalline lens is fitted into the anterior surface of the ball (Fig. 317 l). It is a lenticular, bi-convex, transparent body, the most important of the refractive media in the eye. Of this group we have, besides the corpus vitreum and the lens, the watery fluid (humor aqueus) that is found in front of the lens (at the letter m in Fig. 317). These three transparent refractive media, by which the rays of light that enter the eye are broken up and re-focussed, are enclosed in a solid round capsule, composed of several different coats, something like the concentric layers of an onion. The outermost and thickest of these envelopes is the white sclerotic coat of the eye. It consists of tough white connective tissue. In front of the lens a circular, strongly-curved, transparent plate is fitted into the sclerotic, like the glass of a watch—the cornea (b). At its outer surface the cornea is covered with a very thin layer of the epidermis; this is known as the conjunctiva. It goes from the cornea over the inner surface of the eye-lids, the upper and lower folds which we draw over the eye in closing it. At the inner corner of the eye we have a rudimentary organ in the shape of the relic of a third (inner) eye-lid, which is greatly developed, as “nictitating (winking) membrane,” in the lower Vertebrates (p. 5). Underneath the upper eye-lid are the lachrymal glands, the product of which, the lachrymal fluid, keeps the outer surface of the eye smooth and clean.
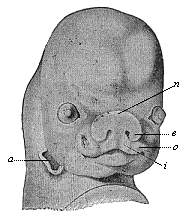
Fig. 315—Face of a human embryo, seven weeks old. (From Kollmann.) Joining of the nasal processes (e outer, i inner) with the upper-jaw process (o), n nasal wall, a ear-opening.
Immediately under the sclerotic we find a very delicate, dark-red membrane, very rich in blood-vessels—the choroid coat—and inside this the retina (o), the expansion of the optic nerve (i). The latter is the second cerebral nerve. It proceeds from the optic thalami (the second cerebral vesicle) to the eye; penetrates its outer envelopes, and then spreads out like a net between the choroid and the corpus vitreum. Between the retina and the choroid there is a very delicate membrane, which is usually (but wrongly) associated with the latter. This is the black pigment-membrane (n). It consists of a single stratum of graceful, hexagonal, regularly-joined cells, full of granules of black colouring matter. This pigment membrane clothes, not only the inner surface of the choroid proper, but also the hind surface of its anterior muscular continuation, which covers the edge of the lens in front as a circular membrane, and arrests the rays of light at the sides. This is the well-known iris of the eye (h), coloured differently in different individuals (blue, grey, brown, etc.); it forms the anterior border of the choroid. The circular opening that is left in the middle is the pupil, through which the rays of light penetrate into the eye. At the point where the iris leaves the anterior border of the choroid proper the latter is very thick, and forms a delicate crown of folds (g), which surrounds the edge of the lens with about seventy large and many smaller rays (corona ciliaris.)
At a very early stage a couple of pear-shaped vesicles develop from the foremost part of the first cerebral vesicle in the embryo of man and the other Craniotes (Figs. 155 a, 297 au). These growths are the primary optic vesicles. They are at first directed outwards and forwards, but presently grow downward, so that, after the complete separation of the five cerebral vesicles, they lie at the base of the intermediate brain. The inner cavities of these pear-shaped vesicles, which soon attain a considerable size, are openly connected with the ventricle of the intermediate brain by their hollow stems. They are covered externally by the epidermis.
At the point where this comes into direct contact with the most curved part of the primary optic vesicle there is a thickening (l) and also a depression (o) of the horny plate (Fig. 318, I). This pit, which we may call the lens-pit, is converted into a closed sac, the thick- walled lens-vesicle (2, l), the thick edges of the pit joining together above it. In the same way in which the medullary tube separates from the outer germinal layer, we now see this lens-sac sever itself entirely from the horny plate (h), its source of origin. The hollow of the sac is afterwards filled with the cells of its thick walls, and thus we get the solid crystalline lens. This is, therefore, a purely epidermic structure. Together with the lens the small underlying piece of corium-plate also separates from the skin.
As the lens separates from the corneous plate and grows inwards, it necessarily hollows out the contiguous primary optic vesicle (Fig. 318, 1–3). This is done in just the same way as the invagination of the blastula, which gives rise to the gastrula in the amphioxus (Fig. 38 C–F). In both cases the hollowing of the closed vesicle on one side goes so far that at last the inner, folded part touches the outer, not folded part, and the cavity disappears. As in the gastrula the first part is converted into the entoderm and the latter into the ectoderm, so in the invagination of the primary optic vesicle the retina (r) is formed from the first (inner) part, and the black pigment membrane (u) from the latter (outer, non-invaginated) part. The hollow stem of the primary optic vesicle is converted into the optic nerve. The lens (l), which has so important a part in this process, lies at first directly on the invaginated part, or the retina (r). But they soon separate, a new structure, the corpus vitreum (gl), growing between them. While the lenticular sac is being detached and is causing the invagination of the primary optic vesicle, another invagination is taking place from below; this proceeds from the superficial part of the skin-fibre layer—the corium of the head. Behind and under the lens a last-shaped process rises from the cutis-plate (Fig. 319 g), hollows out the cup-shaped optic vesicle from below, and presses between the lens (l) and the retina (i). In this way the optic vesicle acquires the form of a hood.
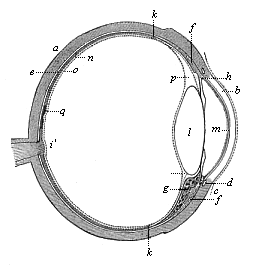
Fig. 317—The human eye in section. a sclerotic coat, b cornea, c conjunctiva, d circular veins of the iris, e choroid coat, f ciliary muscle, g corona ciliaris, h iris, i optic nerve, k anterior border of the retina, l crystalline lens, m inner covering of the cornea (aqueous membrane), n pigment membrane, o retina, p Petit’s canal, q yellow spot of the retina. (From Helmholtz.)
Finally, a complete fibrous envelope, the fibrous capsule of the eye-ball, is formed about the secondary optic vesicle and its stem (the secondary optic nerve). It originates from the part of the head-plates which immediately encloses the eye. This fibrous envelope takes the form of a closed round vesicle, surrounding the whole of the ball and pushing between the lens and the horny plate at its outer side. The round wall of the capsule soon divides into two different membranes by surface-cleavage. The inner membrane becomes the choroid or vascular coat, and in front the ciliary corona and iris. The outer membrane is converted into the white protective or sclerotic coat—in front, the transparent cornea. The eye is now formed in all its essential parts. The further development—the complicated differentiation and composition of the various parts—is a matter of detail.
The chief point in this remarkable evolution of the eye is the circumstance that the optic nerve, the retina, and the pigment membrane originate really from a part of the brain—an outgrowth of the intermediate brain—while the lens, the chief refractive body, develops from the outer skin. From the skin—the horny plate—also arises the delicate conjunctiva, which afterwards covers the outer surface of the eyeball. The lachrymal glands are ramified growths from the conjunctiva (Fig. 286). All these important parts of the eye are products of the outer germinal layer. The remaining parts—the corpus vitreum (with the vascular capsule of the lens), the choroid (with the iris), and the sclerotic (with the cornea)—are formed from the middle germinal layer.
Fig. 318—Eye of the chick embryo in longitudinal section (1. from an embryo sixty-five hours old; 2. from a somewhat older embryo; 3. from an embryo four days old). h horny plate, o lens-pit, l lens (in 1. still part of the epidermis, in 2. and 3. separated from it), x thickening of the horny plate at the point where the lens has severed itself, gl corpus vitreum, r retina, u pigment membrane. (From Remak.)
The outer protection of the eye, the eye-lids, are merely folds of the skin, which are formed in the third month of human embryonic life. In the fourth month the upper eye-lid reaches the lower, and the eye remains covered with them until birth. As a rule, they open wide shortly before birth (sometimes only after birth). Our craniote ancestors had a third eye-lid, the nictitating membrane, which was drawn over the eye from its inner angle. It is still found in many of the Selachii and Amniotes. In the apes and man it has degenerated, and there is now only a small relic of it at the inner corner of the eye, the semi-lunar fold, a useless rudimentary organ (cf. p. 32). The apes and man have also lost the Harderian gland that opened under the nictitating membrane; we find this in the rest of the mammals, and the birds, reptiles, and amphibia.
The peculiar embryonic development of the vertebrate eye does not enable us to draw any definite conclusions as to its obscure phylogeny; it is clearly cenogenetic to a great extent, or obscured by the reduction and curtailment of its original features. It is probable that many of the earlier stages of its phylogeny have disappeared without leaving a trace. It can only be said positively that the peculiar ontogeny of the complicated optic apparatus in man follows just the same laws as in all the other Vertebrates. Their eye is a part of the fore brain, which has grown forward towards the skin, not an original cutaneous sense-organ, as in the Invertebrates.
Fig. 319—Horizontal transverse section of the eye of a human embryo, four weeks old. (From Kölliker.) t lens (the dark wall of which is as thick as the diameter of the central cavity), g corpus vitreum (connected by a stem, g, with the corium), v vascular loop (pressing behind the lens inside the corpus vitreum by means of this stem g), i retina (inner thicker, invaginated layer of the primary optic vesicle), a pigment membrane (outer, thin, non-invaginated layer of same), h space between retina and pigment membrane (remainder of the cavity of the primary optic vesicle).
The vertebrate ear resembles the eye and nose in many important respects, but is different in others, in its development. The auscultory organ in the fully-developed man is like that of the other mammals, and especially the apes, in the main features. As in them, it consists of two chief parts—an apparatus for conducting sound (external and middle ear) and an apparatus for the sensation of sound (internal ear). The external ear opens in the shell at the side of the head (Fig. 320 a). From this point the external passage (b), about an inch in length, leads into the head. The inner end of it is closed by the tympanum, a vertical, but not quite upright, thin membrane of an oval shape (c). This tympanum separates the external passage from the tympanic cavity (d). This is a small cavity, filled with air, in the temporal bone; it is connected with the mouth by a special tube. This tube is rather longer, but much narrower, than the outer passage, leads inwards obliquely from the anterior wall of the tympanic cavity, and opens in the throat below, behind the nasal openings. It is called the Eustachian tube (e); it serves to equalise the pressure of the air within the tympanic cavity and the outer atmosphere that enters by the external passage. Both the Eustachian tube and the tympanic cavity are lined with a thin mucous coat, which is a direct continuation of the mucous lining of the throat. Inside the tympanic cavity there are three small bones which are known (from their shape) as the hammer, anvil, and stirrup (Fig. 320, f, g, h). The hammer (f) is the outermost, next to the tympanum. The anvil (g) fits between the other two, above and inside the hammer. The stirrup (h) lies inside the anvil, and touches with its base the outer wall of the internal ear, or auscultory vesicle. All these parts of the external and middle ear belong to the apparatus for conducting sound. Their chief task is to convey the waves of sound through the thick wall of the head to the inner-lying auscultory vesicle. They are not found at all in the fishes. In these the waves of sound are conveyed directly by the wall of the head to the auscultory vesicle.
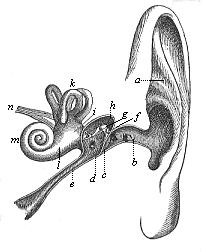
Fig. 320—The human ear (left ear, seen from the front), a shell of ear, b external passage, c tympanum, d tympanic cavity, e Eustachian tube, f, g, h the three bones of the ear (f hammer, g anvil, h stirrup), i utricle, k the three semi-circular canals, l the sacculus, m cochlea, n auscultory nerve.
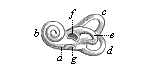
Fig. 321—The bony labyrinth of the human ear (left side). a vestibulum, b cochlea, c upper canal, d posterior canal, e outer canal, f oval fenestra, g round fenestra. (From Meyer.)
The internal apparatus for the sensation of sound, which receives the waves of sound from the conducting apparatus, consists in man and all other mammals of a closed auscultory vesicle filled with fluid and an auditory nerve, the ends of which expand over the wall of this vesicle. The vibrations of the sound-waves are conveyed by these media to the nerve-endings. In the labyrinthic water that fills the auscultory vesicle there are small stones at the points of entry of the acoustic nerves, which are composed of groups of microscopic calcareous crystals (otoliths). The auscultory organ of most of the Invertebrates has substantially the same composition. It usually consists of a closed vesicle, filled with fluid, and containing otoliths, with the acoustic nerve expanding on its wall. But, while the auditory vesicle is usually of a simple round or oval shape in the Invertebrates, it has in the Vertebrates a special and curious structure, the labyrinth. This thin-membraned labyrinth is enclosed in a bony capsule of the same shape, the osseous labyrinth (Fig. 321), and this lies in the middle of the petrous bone of the skull. The labyrinth is divided into two vesicles in all the Gnathostomes. The larger one is called the utriculus, and has three arched appendages, called the “semi-circular canals” (c, d, e). The smaller vesicle is called the sacculus, and is connected with a peculiar appendage, with (in man and the higher mammals) a spiral form something like a snail’s shell, and therefore called the cochlea (= snail, b). On the thin wall of this delicate labyrinth the acoustic nerve, which comes from the after-brain, spreads out in most elaborate fashion. It divides into two main branches—a cochlear nerve (for the cochlea) and a vestibular nerve (for the rest of the labyrinth). The former seems to have more to do with the quality, the latter with the quantity, of the acoustic sensations. Through the cochlear nerves we learn the height and timbre, through the vestibular nerves the intensity, of tones.
The first structure of this highly elaborate organ is very simple in the embryo of man and all the other Craniotes; it is a pit-like depression in the skin. At the back part of the head at both sides, near the after brain, a small thickening of the horny plate is formed at the upper end of the second gill-cleft (Fig. 322 A fl). This sinks into a sort of pit, and severs from the epidermis, just as the lens of the eye does. In this way is formed at each side, directly under the horny plate of the back part of the head, a small vesicle filled with fluid, the primitive auscultory vesicle, or the primary labyrinth. As it separates from its source, the horny plate, and presses inwards and backwards into the skull, it changes from round to pear-shaped (Figs. 322 B lv, 323 o). The outer part of it is lengthened into a thin stem, which at first still opens outwards by a narrow canal. This is the labyrinthic appendage (Fig. 322 lr). In the lower Vertebrates it develops into a special cavity filled with calcareous crystals, which remains open permanently in some of the primitive fishes, and opens outwards in the upper part of the skull. But in the mammals the labyrinthic appendage degenerates. In these it has only a phylogenetic interest as a rudimentary organ, with no actual physiological significance. The useless relic of it passes through the wall of the petrous bone in the shape of a narrow canal, and is called the vestibular aqueduct.
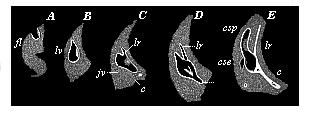
Fig. 322—Development of the auscultory labyrinth of the chick, in five successive stages (A–E). (Vertical transverse sections of the skull.) fl auscultory pits, lv auscultory vesicles, lr labyrinthic appendage, c rudimentary cochlea, csp posterior canal, cse external canal, jv jugular vein. (From Reissner.)
It is only the inner and lower bulbous part of the separated auscultory vesicle that develops into the highly complex and differentiated structure that is afterwards known as the secondary labyrinth. This vesicle divides at an early stage into an upper and larger and a lower and smaller section. From the one we get the utriculus with the semi-circular canals; from the other the sacculus and the cochlea (Fig. 320 c). The canals are formed in the shape of simple pouch-like involutions of the utricle (cse and csp). The edges join together in the middle part of each fold, and separate from the utricle, the two ends remaining in open connection with its cavity. All the Gnathostomes have these three canals like man, whereas among the Cyclostomes the lampreys have only two and the hag-fishes only one. The very complex structure of the cochlea, one of the most elaborate and wonderful outcomes of adaptation in the mammal body, develops originally in very simple fashion as a flask-like projection from the sacculus. As Hasse and Retzius have pointed out, we find the successive ontogenetic stages of its growth represented permanently in the series of the higher Vertebrates. The cochlea is wanting even in the Monotremes, and is restricted to the rest of the mammals and man.
The auditory nerve, or eighth cerebral nerve, expands with one branch in the cochlea, and with the other in the remaining parts of the labyrinth. This nerve is, as Gegenbaur has shown, the sensory dorsal branch of a cerebro-spinal nerve, the motor ventral branch of which acts for the muscles of the face (nervus facialis). It has therefore originated phylogenetically from an ordinary cutaneous nerve, and so is of quite different origin from the optic and olfactory nerves, which both represent direct outgrowths of the brain. In this respect the auscultory organ is essentially different from the organs of sight and smell. The acoustic nerve is formed from ectodermic cells of the hind brain, and develops from the nervous structure that appears at its dorsal limit. On the other hand, all the membranous, cartilaginous, and osseous coverings of the labyrinth are formed from the mesodermic head-plates.
The apparatus for conducting sound which we find in the external and middle ear of mammals develops quite separately from the apparatus for the sensation of sound. It is both phylogenetically and ontogenetically an independent secondary formation, a later accession to the primary internal ear. Nevertheless, its development is not less interesting, and is explained with the same ease by comparative anatomy. In all the fishes and in the lowest Vertebrates there is no special apparatus for conducting sound, no external or middle ear; they have only a labyrinth, an internal ear, which lies within the skull. They are without the tympanum and tympanic cavity, and all its appendages. From many observations made in the last few decades it seems that many of the fishes (if not all) cannot distinguish tones; their labyrinth seems to be chiefly (if not exclusively) an organ for the sense of space (or equilibrium). If it is destroyed, the fishes lose their balance and fall. In the opinion of recent physiologists this applies also to many of the Invertebrates (including the nearer ancestors of the Vertebrates). The round vesicles which are considered to be their auscultory vesicles, and which contain an otolith, are supposed to be merely organs of the sense of space (“static vesicles or statocysts”).
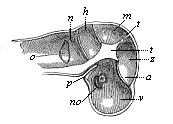
Fig. 323—Primitive skull of the human embryo, four weeks old, vertical section, left half seen internally. v, z, m, h, n the five pits of the cranial cavity, in which the five cerebral vesicles lie (fore, intermediate, middle, hind, and after brains), o pear-shaped primary auscultory vesicle (appearing through), a eye (appearing through), no optic nerve, p canal of the hypophysis, t central prominence of the skull. (From Kölliker.)
The middle ear makes its first appearance in the amphibian class, where we find a tympanum, tympanic cavity, and Eustachian tube; these animals, and all terrestrial Vertebrates, certainly have the faculty of hearing. All these essential parts of the middle ear originate from the first gill-cleft and its surrounding part; in the Selachii this remains throughout life an open squirting-hole, and lies between the first and second gill-arch. In the embryo of the higher Vertebrates it closes up in the centre, and thus forms the tympanic membrane. The outlying remainder of the first gill-cleft is the rudiment of the external meatus. From its inner part we get the tympanic cavity, and, further inward still, the Eustachian tube. Connected with this is the development of the three bones of the mammal ear from the first two gill-arches; the hammer and anvil are formed from the first, the stirrup from the upper end of the second, gill-arch.
Fig. 324—The rudimentary muscles of the ear in the human skull. a raising muscle (M. attollens), b drawing muscle (M. attrahens), c withdrawing muscle (M. retrahens), d large muscle of the helix (M. helicis major), e small muscle of the helix (M. helicis minor), f muscle of the angle of the ear (M. tragicus), g anti-angular muscle (M. antitragicus). (From H. Meyer.)
Finally, the shell (pinna or concha) and external meatus (passage to the tympanum) of the outer ear are developed in a very simple fashion from the skin that borders the external aperture of the first gill-cleft. The shell rises in the shape of a circular fold of the skin, in which cartilage and muscles are afterwards formed (Figs. 313, 315). This organ is only found in the mammalian class. It is very rudimentary in the lowest section, the Monotremes. In the others it is found at very different stages of development, and sometimes of degeneration. It is degenerate in most of the aquatic mammals. The majority of them have lost it altogether—for instance, the walruses and whales and most of the seals. On the other hand, the pinna is well developed in the great majority of the Marsupials and Placentals; it receives and collects the waves of sound, and is equipped with a very elaborate muscular apparatus, by means of which the pinna can be turned freely in any direction and its shape be altered. It is well known how readily domestic animals—horses, cows, dogs, hares, etc.—point their ears and move them in different directions. Most of the apes do the same, and our earlier ape ancestors were also able to do it. But our later simian ancestors, which we have in common with the anthropoid apes, abandoned the use of these muscles, and they gradually became rudimentary and useless. However, we possess them still (Fig. 324). In fact, some men can still move their ears a little backward and forward by means of the drawing and withdrawing muscles (b and c); with practice this faculty can be much improved. But no man can now lift up his ears by the raising muscle (a), or change the shape of them by the small inner muscles (d, e, f, g). These muscles were very useful to our ancestors, but are of no consequence to us. This applies to most of the anthropoid apes as well.
We also share with the higher anthropoid apes (gorilla, chimpanzee, and orang) the characteristic form of the human outer ear, especially the folded border, the helix and the lobe. The lower apes have pointed ears, without folded border or lobe, like the other mammals. But Darwin has shown that at the upper part of the folded border there is in many men a small pointed process, which most of us do not possess. In some individuals this process is well developed. It can only be explained as the relic of the original point of the ear, which has been turned inwards in consequence of the curving of the edge. If we compare the pinna of man and the various apes in this respect, we find that they present a connected series of degenerate structures. In the common catarrhine ancestors of the anthropoids and man the degeneration set in with the folding together of the pinna. This brought about the helix of the ear, in which we find the significant angle which represents the relic of the salient point of the ear in our earlier simian ancestors. Here again, therefore, comparative anatomy enables us to trace with certainty the human ear to the similar, but more developed, organ of the lower mammals. At the same time, comparative physiology shows that it was a more or less useful implement in the latter, but it is quite useless in the anthropoids and man. The conducting of the sound has scarcely been affected by the loss of the pinna. We have also in this the explanation of the extraordinary variety in the shape and size of the shell of the ear in different men; in this it resembles other rudimentary organs.
The peculiar structure of the locomotive apparatus is one of the features that are most distinctive of the vertebrate stem. The chief part of this apparatus is formed, as in all the higher animals, by the active organs of movement, the muscles; in consequence of their contractility they have the power to draw up and shorten themselves. This effects the movement of the various parts of the body, and thus the whole body is conveyed from place to place. But the arrangement of these muscles and their relation to the solid skeleton are different in the Vertebrates from the Invertebrates.
In most of the lower animals, especially the Platodes and Vermalia, we find that the muscles form a simple, thin layer of flesh immediately underneath the skin. This muscular layer is very closely connected with the skin itself; it is the same in the Mollusc stem. Even in the large division of the Articulates, the classes of crabs, spiders, myriapods, and insects, we find a similar feature, with the difference that in this case the skin forms a solid armour—a rigid cutaneous skeleton made of chitine (and often also of carbonate of lime). This external chitine coat undergoes a very elaborate articulation both on the trunk and the limbs of the Articulates, and in consequence the muscular system also, the contractile fibres of which are attached inside the chitine tubes, is highly articulated. The Vertebrates form a direct contrast to this. In these alone a solid internal skeleton is developed, of cartilage or bone, to which the muscles are attached. This bony skeleton is a complex lever apparatus, or passive apparatus of movement. Its rigid parts, the arms of the levers, or the bones, are brought together by the actively mobile muscles, as if by drawing-ropes. This admirable locomotorium, especially its solid central axis, the vertebral column, is a special feature of the Vertebrates, and has given the name to the group.
Fig. 328—A piece of the axial rod (chorda dorsalis), from a sheep embryo. a cuticular sheath, b cells. (From Kölliker.)
In order to get a clear idea of the chief features of the development of the human skeleton, we must first examine its composition in the adult frame (Fig. 325, the human skeleton seen from the right; Fig. 326, front view of the whole skeleton). As in other mammals, we distinguish first between the axial or dorsal skeleton and the skeleton of the limbs. The axial skeleton consists of the vertebral column (the skeleton of the trunk) and the skull (skeleton of the head); the latter is a peculiarly modified part of the former. As appendages of the vertebral column we have the ribs, and of the skull we have the hyoid bone, the lower jaw, and the other products of the gill-arches.
The skeleton of the limbs or extremities is composed of two groups of parts—the skeleton of the extremities proper and the zone-skeleton, which connects these with the vertebral column. The zone-skeleton of the arms (or fore legs) is the shoulder-zone; the zone-skeleton of the legs (or hind legs) is the pelvic zone.
The vertebral column (Fig. 327) in man is composed of thirty-three to thirty-five ring-shaped bones in a continuous series (above each other, in man’s upright position). These vertebræ are separated from each other by elastic ligaments, and at the same time connected by joints, so that the whole column forms a firm and solid, but flexible and elastic, axial skeleton, moving freely in all directions. The vertebræ differ in shape and connection at the various parts of the trunk, and we distinguish the following groups in the series, beginning at the top: Seven cervical vertebræ, twelve dorsal vertebræ, five lumbar vertebræ, five sacral vertebræ, and four to six caudal vertebræ. The uppermost, or those next to the skull, are the cervical vertebræ (Fig. 327); they have a hole in each of the lateral processes. There are seven of these vertebræ in man and almost all the other mammals, even if the neck is as long as that of the camel or giraffe, or as short as that of the mole or hedgehog. This constant number, which has few exceptions (due to adaptation), is a strong proof of the common descent of the mammals; it can only be explained by faithful heredity from a common stem-form, a primitive mammal with seven cervical vertebræ. If each species had been created separately, it would have been better to have given the long-necked mammals more, and the short-necked animals less, cervical vertebræ. Next to these come the dorsal (or pectoral) vertebræ, which number twelve to thirteen (usually twelve) in man and most of the other mammals. Each dorsal vertebra (Fig. 165) has at the side, connected by joints, a couple of ribs, long bony arches that lie in and protect the wall of the chest. The twelve pairs of ribs, together with the connecting intercostal muscles and the sternum, which joins the ends of the right and left ribs in front, form the chest (thorax). In this strong and elastic frame are the lungs, and between them the heart. Next to the dorsal vertebræ comes a short but stronger section of the column, formed of five large vertebræ. These are the lumbar vertebræ (Fig. 166); they have no ribs and no holes in the transverse processes. To these succeeds the sacral bone, which is fitted between the two halves of the pelvic zone. The sacrum is formed of five vertebræ, completely blended together. Finally, we have at the end a small rudimentary caudal column, the coccyx. This consists of a varying number (usually four, more rarely three, or five or six) of small degenerated vertebræ, and is a useless rudimentary organ with no actual physiological significance. Morphologically, however, it is of great interest as an irrefragable proof of the descent of man and the anthropoids from long-tailed apes. On no other theory can we explain the existence of this rudimentary tail. In the earlier stages of development the tail of the human embryo protrudes considerably. It afterwards atrophies; but the relic of the atrophied caudal vertebræ and of the rudimentary muscles that once moved it remains permanently. Sometimes, in fact, the external tail is preserved. The older anatomists say that the tail is usually one vertebra longer in the human female than in the male (or four against five); Steinbach says it is the reverse.
Fig. 329—Three dorsal vertebræ, from a human embryo, eight weeks old, in lateral longitudinal section. v cartilaginous vertebral body, li inter-vertebral disks, ch chorda. (From Kölliker.)
Fig. 330—A dorsal vertebra of the same embryo, in lateral transverse section. cv cartilaginous vertebral body, ch chorda, pr transverse process, a vertebral arch (upper arch), c upper end of the rib (lower arch). (From Kölliker.)
In the human vertebral column there are usually thirty-three vertebræ. It is interesting to find, however, that the number often changes, one or two vertebræ dropping out or an additional one appearing. Often, also, a mobile rib is formed at the last cervical or the first lumbar vertebra, so that there are then thirteen dorsal vertebræ, besides six cervical and four lumbar. In this way the contiguous vertebræ of the various sections of the column may take each other’s places.
In order to understand the embryology of the human vertebral column we must first carefully consider the shape and connection of the vertebræ. Each vertebra has, in general, the shape of a seal-ring (Figs. 164–166). The thicker portion, which is turned towards the ventral side, is called the body of the vertebra, and forms a short osseous disk; the thinner part forms a semi-circular arch, the vertebral arch, and is turned towards the back. The arches of the successive vertebræ are connected by thin intercrural ligaments in such a way that the cavity they collectively enclose represents a long canal. In this vertebral canal we find the trunk part of the central nervous system, the spinal cord. Its head part, the brain, is enclosed by the skull, and the skull itself is merely the uppermost part of the vertebral column, distinctively modified. The base or ventral side of the vesicular cranial capsule corresponds originally to a number of developed vertebral bodies; its vault or dorsal side to their combined upper vertebral arches.
While the solid, massive bodies of the vertebræ represent the real central axis of the skeleton, the dorsal arches serve to protect the central marrow they enclose. But similar arches develop on the ventral side for the protection of the viscera in the breast and belly. These lower or ventral vertebral arches, proceeding from the ventral side of the vertebral bodies, form, in many of the lower Vertebrates, a canal in which the large blood-vessels are enclosed on the lower surface of the vertebral column (aorta and caudal vein). In the higher Vertebrates the majority of these vertebral arches are lost or become rudimentary. But at the thoracic section of the column they develop into independent strong osseous arches, the ribs (costæ). In reality the ribs are merely large and independent lower vertebral arches, which have lost their original connection with the vertebral bodies.
Fig. 331—Intervertebral disk of a new-born infant, transverse section. a rest of the chorda. (From Kölliker.)
If we turn from this anatomic survey of the composition of the column to the question of its development, I may refer the reader to earlier pages with regard to the first and most important points (pp. 145–148). It will be remembered that in the human embryo and that of the other vertebrates we find at first, instead of the segmented column, only a simple unarticulated cartilaginous rod. This solid but flexible and elastic rod is the axial rod (or the chorda dorsalis). In the lowest Vertebrate, the Amphioxus, it retains this simple form throughout life, and permanently represents the whole internal skeleton (Fig. 210 i). In the Tunicates, also, the nearest Invertebrate relatives of the Vertebrates, we meet the same chorda—transitorily in the passing larva tail of the Ascidia, permanently in the Copelata (Fig. 225 c). Undoubtedly both the Tunicates and Acrania have inherited the chorda from a common unsegmented stem-form; and these ancient, long-extinct ancestors of all the chordonia are our hypothetical Prochordonia.
Long before there is any trace of the skull, limbs, etc., in the embryo of man or any of the higher Vertebrates—at the early stage in which the whole body is merely a sole-shaped embryonic shield—there appears in the middle line of the shield, directly under the medullary furrow, the simple chorda. (Cf. Figs. 131–135 ch). It follows the long axis of the body in the shape of a cylindrical axial rod of elastic but firm composition, equally pointed at both ends. In every case the chorda originates from the dorsal wall of the primitive gut; the cells that compose it (Fig. 328 b) belong to the entoderm (Figs. 216–221). At an early stage the chorda develops a transparent structureless sheath, which is secreted from its cells (Fig. 328 a). This chordalemma is often called the “inner chorda-sheath,” and must not be confused with the real external sheath, the mesoblastic perichorda.
But this unsegmented primary axial skeleton is soon replaced by the segmented secondary axial skeleton, which we know as the vertebral column. The provertebral plates (Fig. 124 s) differentiate from the innermost, median part of the visceral layer of the cœlom-pouches at each side of the chorda. As they grow round the chorda and enclose it they form the skeleton plate or skeletogenetic layer—that is to say, the skeleton-forming stratum of cells, which provides the mobile foundation of the permanent vertebral column and skull (scleroblast). In the head-half of the embryo the skeletal plate remains a continuous, simple, undivided layer of tissue, and presently enlarges into a thin-walled capsule enclosing the brain, the primordial skull. In the trunk-half the provertebral plate divides into a number of homogeneous, cubical, successive pieces; these are the several primitive vertebræ. They are not numerous at first, but soon increase as the embryo grows longer (Figs. 153–155).

Fig. 333—Skull of a new-born child. (From Kollmann.) Above, in the three bones of the roof of the skull, we see the lines that radiate from the central points of ossification; in front, the frontal bone; behind, the occipital bone; between the two the large parietal bone, p. s the scurf bone, w mastoid fontanelle, f petrous bone, t tympanic bone, l lateral part, b bulla, j cheek-bone, a large wing of cuneiform bone, k fontanelle of cuneiform bone.
In all the Craniotes the soft, indifferent cells of the mesoderm, which originally compose the skeletal plate, are afterwards converted for the most part into cartilaginous cells, and these secrete a firm and elastic intercellular substance between them, and form cartilaginous tissue. Like most of the other parts of the skeleton, the membranous rudiments of the vertebræ soon pass into a cartilaginous state, and in the higher Vertebrates this is afterwards replaced by the hard osseous tissue with its characteristic stellate cells (Fig. 6). The primary axial skeleton remains a simple chorda throughout life in the Acrania, the Cyclostomes, and the lowest fishes. In most of the other Vertebrates the chorda is more or less replaced by the cartilaginous tissue of the secondary perichorda that grows round it. In the lower Craniotes (especially the fishes) a more or less considerable part of the chorda is preserved in the bodies of the vertebræ. In the mammals it disappears for the most part. By the end of the second month in the human embryo the chorda is merely a slender thread, running through the axis of the thick, cartilaginous vertebral column (Figs. 182 ch, 329 ch). In the cartilaginous vertebral bodies themselves, which afterwards ossify, the slender remnant of the chorda presently disappears (Fig. 330 ch). But in the elastic inter-vertebral disks, which develop from the skeletal plate between each pair of vertebral bodies (Fig. 329 li), a relic of the chorda remains permanently. In the new-born child there is a large pear-shaped cavity in each intervertebral disk, filled with a gelatinous mass of cells (Fig. 331 a).
Fig. 334—Head-skeleton of a primitive fish. n nasal pit, eth cribriform bone region, orb orbit of eye, la wall of auscultory labyrinth, occ occipital region of primitive skull, cv vertebral column, a fore, bc hind-lip cartilage, o primitive upper jaw (palato-quadratum), u primitive lower jaw, II hyaloid bone, III–VIII first to sixth branchial arches. (From Gegenbaur.)
Though less sharply defined, this gelatinous nucleus of the elastic cartilaginous disks persists throughout life in the mammals, but in the birds and most reptiles the last trace of the chorda disappears. In the subsequent ossification of the cartilaginous vertebra the first deposit of bony matter (“first osseous nucleus”) takes place in the vertebral body immediately round the remainder of the chorda, and soon displaces it altogether. Then there is a special osseous nucleus formed in each half of the vertebral arch. The ossification does not reach the point at which the three nuclei are joined until after birth. In the first year the two osseous halves of the arches unite; but it is much later—in the second to the eighth year— that they connect with the osseous vertebral bodies.
Fig. 335—Roofs of the skulls of nine Primates (Cattarrhines), seen from above and reduced to a common size. 1 European, 2 Brazilian, 3 Pithecanthropus, 4 Gorilla, 5 Chimpanzee, 6 Orang, 7 Gibbon, 8 Tailed ape, 9 Baboon.
The bony skull (cranium), the head-part of the secondary axial skeleton, develops in just the same way as the vertebral column. The skull forms a bony envelope for the brain, just as the vertebral canal does for the spinal cord; and as the brain is only a peculiarly differentiated part of the head, while the spinal cord represents the longer trunk-section of the originally homogeneous medullary tube, we shall expect to find that the osseous coat of the one is a special modification of the osseous envelope of the other. When we examine the adult human skull in itself (Fig. 332), it is difficult to conceive how it can be merely the modified fore part of the vertebral column. It is an elaborate and extensive bony structure, composed of no less than twenty bones of different shapes and sizes. Seven of them form the spacious shell that surrounds the brain, in which we distinguish the solid ventral base below and the curved dorsal vault above. The other thirteen bones form the facial skull, which is especially the bony envelope of the higher sense-organs, and at the same time encloses the entrance of the alimentary canal. The lower jaw is articulated at the base of the skull (usually regarded as the XXI cranial bone). Behind the lower jaw we find the hyoid bone at the root of the tongue, also formed from the gill-arches, and a part of the lower arches that have developed as “head-ribs” from the ventral side of the base of the cranium.
Fig. 336—Skeleton of the breast-fin of
Ceratodus (biserial feathered skeleton). A, B, cartilaginous series
of the fin-stem. rr cartilaginous fin-radii. (From Gunther.)
Fig. 337—Skeleton of the breast-fin of an early Selachius
(Acanthias). The radii of the median fin-border (B) have
disappeared for the most part; a few only (R) are left. R, R,
radii of the lateral fin-border, mt metapterygium, ms
mesopterygium, p propterygium. (From Gegenbaur.)
Fig.
338—Skeleton of the breast-fin of a young Selachius. The radii of
the median fin-border have wholly disappeared. The shaded part on the right is
the section that persists in the five-fingered hand of the higher Vertebrates.
(b the three basal pieces of the fin: mt metapterygium, rudiment
of the humerus, ms mesopterygium, p propterygium.) (From
Gegenbaur.)
Although the fully-developed skull of the higher Vertebrates, with its peculiar shape, its enormous size, and its complex composition, seems to have nothing in common with the ordinary vertebræ, nevertheless even the older comparative anatomists came to recognise at the end of the eighteenth century that it is really nothing else originally than a series of modified vertebræ. When Goethe in 1790 “picked up the skull of a slain victim from the sand of the Jewish cemetery at Venice, he noticed at once that the bones of the face also could be traced to vertebræ (like the three hind-most cranial vertebræ).” And when Oken (without knowing anything of Goethe’s discovery) found at Ilenstein, “a fine bleached skull of a hind, the thought flashed across him like lightning: ‘It is a vertebral column.’”
This famous vertebral theory of the skull has interested the most distinguished zoologists for more than a century: the chief representatives of comparative anatomy have devoted their highest powers to the solution of the problem, and the interest has spread far beyond their circle. But it was not until 1872 that it was happily solved, after seven years’ labour, by the comparative anatomist who surpassed all other experts of this science in the second half of the nineteenth century by the richness of his empirical knowledge and the acuteness and depth of his philosophic speculations. Carl Gegenbaur has shown, in his classic Studies of the Comparative Anatomy of the Vertebrates (third section), that we find the most solid foundation for the vertebral theory of the skull in the head-skeleton of the Selachii. Earlier anatomists had wrongly started from the mammal skull, and had compared the several bones that compose it with the several parts of the vertebra (Fig. 333) they thought they could prove in this way that the fully-formed mammal skull was made of from three to six vertebræ.
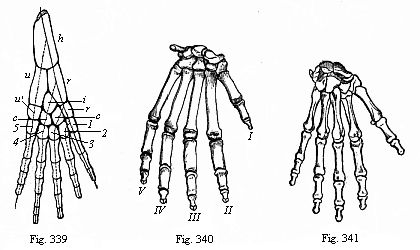
Fig. 339—Skeleton of the fore leg of an
amphibian. h upper-arm (humerus), ru lower arm (r
radius, u ulna), rcicu′, wrist-bones of first series
(r radiale, i intermedium, c centrale, u′
ulnare). 1, 2, 3, 4, 5 wrist-bones of the second series. (From
Gegenbaur.)
Fig. 340—Skeleton of gorilla’s hand. (From
Huxley.)
Fig. 341—Skeleton of human hand, back. (From
Meyer.)
The older theory was refuted by simple and obvious facts, which were first pointed out by Huxley. Nevertheless, the fundamental idea of it—the belief that the skull is formed from the head-part of the perichordal axial skeleton, just as the brain is from the simple medullary tube, by differentiation and modification—remained. The work now was to discover the proper way of supplying this philosophic theory with an empirical foundation, and it was reserved for Gegenbaur to achieve this. He first opened out the phylogenetic path which here, as in all morphological questions, leads most confidently to the goal. He showed that the primitive fishes (Figs. 249–251), the ancestors of all the Gnathostomes, still preserve permanently in the form of their skull the structure out of which the transformed skull of the higher Vertebrates, including man, has been evolved. He further showed that the branchial arches of the Selachii prove that their skull originally consisted of a large number of (at least nine or ten) provertebræ, and that the cerebral nerves that proceed from the base of the brain entirely confirm this. These cerebral nerves are (with the exception of the first and second pair, the olfactory and optic nerves) merely modifications of spinal nerves, and are essentially similar to them in their peripheral expansion. The comparative anatomy of these cerebral nerves, their origin and their expansion, furnishes one of the strongest arguments for the new vertebral theory of the skull.
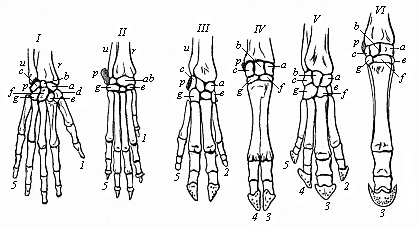
Fig. 342—Skeleton of the hand or fore foot of six mammals. I man, II dog, III pig, IV ox, V tapir, VI horse. r radius, u ulna, a scaphoideum, b lunare, a triquetrum, d trapezium, e trapezoid, f capitatum, g hamatum, p pisiforme. 1 thumb, 2 index finger, 3 middle finger, 4 ring finger, 5 little finger. (From Gegenbaur.)
We have not space here to go into the details of Gegenbaur’s theory of the skull. I must be content to refer the reader to the great work I have mentioned, in which it is thoroughly established from the empirico-philosophical point of view. He has also given a comprehensive and up-to-date treatment of the subject in his Comparative Anatomy of the Vertebrates (1898). Gegenbaur indicates as original “cranial ribs,” or “lower arches of the cranial vertebræ,” at each side of the head of the Selachii (Fig. 334), the following pairs of arches: I and II, two lip-cartilages, the anterior (a) of which is composed of an upper piece only, the posterior (bc) from an upper and lower piece; III, the maxillary arches, also consisting of two pieces on each side—the primitive upper jaw (os palato-quadratum, o) and the primitive lower jaw (u); IV, the hyaloid bone (II); finally, V–X, six branchial arches in the narrower sense (III–VIII). From the anatomic features of these nine to ten cranial ribs or “lower vertebral arches” and the cranial nerves that spread over them, it is clear that the apparently simple cartilaginous primitive skull of the Selachii was originally formed from so many (at least nine) somites or provertebræ. The blending of these primitive segments into a single capsule is, however, so ancient that, in virtue of the law of curtailed heredity, the original division seems to have disappeared; in the embryonic development it is very difficult to detect it in isolated traces, and in some respects quite impossible. It is claimed that several (three to six) traces of provertebræ have been discovered in the anterior (pre-chordal) part of the Selachii-skull; this would bring up the number of cranial somites to twelve or sixteen, or even more.
In the primitive skull of man (Fig. 323) and the higher Vertebrates, which has been evolved from that of the Selachii, five consecutive sections are discoverable at a certain early period of development, and one might be induced to trace these to five primitive vertebræ; but these sections are due entirely to adaptation to the five primitive cerebral vesicles, and correspond, like these, to a large number of metamera. That we have in the primitive skull of the mammals a greatly modified and transformed organ, and not at all a primitive formation, is clear from the circumstance that its original soft membranous form only assumes the cartilaginous character for the most part at the base and the sides, and remains membranous at the roof. At this part the bones of the subsequent osseous skull develop as external coverings over the membranous structure, without an intermediate cartilaginous stage, as there is at the base of the skull. Thus a large part of the cranial bones develop originally as covering bones from the corium, and only secondarily come into close touch with the primitive skull (Fig. 333). We have previously seen how this very rudimentary beginning of the skull in man is formed ontogenetically from the “head-plates,” and thus the fore end of the chorda is enclosed in the base of the skull. (Cf. Fig. 145 and pp. 138, 144, and 149.)
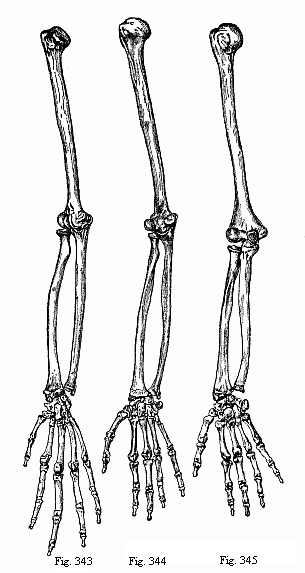
Figs. 343–345—Arm and hand of three anthropoids. Fig. 343—Chimpanzee (Anthropithecus niger). Fig. 344—Veddah of Ceylon (Homo veddalis). Fig. 345—European (Homo mediterraneus). (From Paul and Fritz Sarasin.)
The phylogeny of the skull has made great progress during the last three decades through the joint attainments of comparative anatomy, ontogeny, and paleontology. By the judicious and comprehensive application of the phylogenetic method (in the sense of Gegenbaur) we have found the key to the great and important problems that arise from the thorough comparative study of the skull. Another school of research, the school of what is called “exact craniology” (in the sense of Virchow), has, meantime, made fruitless efforts to obtain this result. We may gratefully acknowledge all that this descriptive school has done in the way of accurately describing the various forms and measurements of the human skull, as compared with those of other mammals. But the vast empirical material that it has accumulated in its extensive literature is mere dead and sterile erudition until it is vivified and illumined by phylogenetic speculation.
Virchow confined himself to the most careful analysis of large numbers of human skulls and those of anthropoid mammals. He saw only the differences between them, and sought to express these in figures.
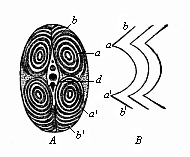
Fig. 346—Transverse section of a fish’s tail (from the tunny). (From Johannes Müller.) a upper (dorsal) lateral muscles, a′, b′ lower (ventral) lateral muscles, d vertebral bodies, b sections of incomplete conical mantle, B attachment lines of the inter-muscular ligaments (from the side).
Without adducing a single solid reason, or offering any alternative explanation, he rejected evolution as an unproved hypothesis. He played a most unfortunate part in the controversy as to the significance of the fossil human skulls of Spy and Neanderthal, and the comparison of them with the skull of the Pithecanthropus (Fig. 283). All the interesting features of these skulls that clearly indicated the transition from the anthropoid to the man were declared by Virchow to be chance pathological variations. He said that the roof of the skull of Pithecanthropus (Fig. 335, 3) must have belonged to an ape, because so pronounced an orbital stricture (the horizontal constriction between the outer edge of the eye-orbit and the temples) is not found in any human being. Immediately afterwards Nehring showed in the skull of a Brazilian Indian (Fig. 335, 2), found in the Sambaquis of Santos, that this stricture can be even deeper in man than in many of the apes. It is very instructive in this connection to compare the roofs of the skulls (seen from above) of different primates. I have, therefore, arranged nine such skulls in Fig. 335, and reduced them to a common size.
We turn now to the branchial arches, which were regarded even by the earlier natural philosophers as “head-ribs.” (Cf. Figs. 167–170). Of the four original gill-arches of the mammals the first lies between the primitive mouth and the first gill-cleft. From the base of this arch is formed the upper-jaw process, which joins with the inner and outer nasal processes on each side, in the manner we have previously explained, and forms the chief parts of the skeleton of the upper jaw (palate bone, pterygoid bone, etc.) (Cf. p. 284.) The remainder of the first branchial arch, which is now called, by way of contrast, the “upper-jaw process,” forms from its base two of the ear-ossicles (hammer and anvil), and as to the rest is converted into a long strip of cartilage that is known, after its discoverer, as “Meckel’s cartilage,” or the promandibula. At the outer surface of the latter is formed from the cellular matter of the corium, as covering or accessory bone, the permanent bony lower jaw. From the first part or base of the second branchial arch we get, in the mammals, the third ossicle of the ear, the stirrup; and from the succeeding parts we get (in this order) the muscle of the stirrup, the styloid process of the temporal bone, the styloid-hyoid ligament, and the little horn of the hyoid bone. The third branchial arch is only cartilaginous at the foremost part, and here the body of the hyoid bone and its larger horn are formed at each side by the junction of its two halves. The fourth branchial arch is only found transitorily in the mammal embryo as a rudimentary organ, and does not develop special parts; and there is no trace in the embryo of the higher Vertebrates of the posterior branchial arches (fifth and sixth pair), which are permanent in the Selachii. They have been lost long ago. Moreover, the four gill-clefts of the human embryo are only interesting as rudimentary organs, and they soon close up and disappear. The first alone (between the first and second branchial arches) has any permanent significance; from it are developed the tympanic cavity and the Eustachian tube. (Cf. Figs. 169, 320.)
It was Carl Gegenbaur again who solved the difficult problem of tracing the skeleton of the limbs of the Vertebrates to a common type. Few parts of the vertebrate body have undergone such infinitely varied modifications in regard to size, shape, and adaptation of structure as the limbs or extremities; yet we are in a position to reduce them all to the same hereditary standard. We may generally distinguish three groups among the Vertebrates in relation to the formation of their limbs. The lowest and earliest Vertebrates, the Acrania and Cyclostomes, had, like their invertebrate ancestors, no pairs of limbs, as we see in the Amphioxus and the Cyclostomes to-day (Figs. 210, 247). The second group is formed of the two classes of the true fishes and the Dipneusts; here there are always two pairs of limbs at first, in the shape of many-toed fins—one pair of breast-fins or fore legs, and one pair of belly-fins or hind legs (Figs. 248–259). The third group comprises the four higher classes of Vertebrates—the amphibia, reptiles, birds, and mammals; in these quadrupeds there are at first the same two pairs of limbs, but in the shape of five-toed feet. Frequently we find less than five toes, and sometimes the feet are wholly atrophied (as in the serpents). But the original stem-form of the group had five toes or fingers before and behind (Figs. 263–265).
The true primitive form of the pairs of limbs, such as they were found in the primitive fishes of the Silurian period, is preserved for us in the Australian dipneust, the remarkable Ceratodus (Fig. 257). Both the breast-fin and the belly-fin are flat oval paddles, in which we find a biserial cartilaginous skeleton (Fig. 336). This consists, firstly, of a much segmented fin-rod or “stem” (A, B), which runs through the fin from base to tip; and secondly of a double row of thin articulated fin-radii (r, r), which are attached to both sides of the fin-rod, like the feathers of a feathered leaf. This primitive fin, which Gegenbaur first recognised, is attached to the vertebral column by a simple zone in the shape of a cartilaginous arch. It has probably originated from the branchial arches.[31]
[31] While Gegenbaur derives the fins from two pairs of posterior separated branchial arches, Balfour holds that they have been developed from segments of a pair of originally continuous lateral fins or folds of the skin.)
We find the same biserial primitive fin more or less preserved in the fossilised remains of the earliest Selachii (Fig. 248), Ganoids (Fig. 253), and Dipneusts (Fig. 256). It is also found in modified form in some of the actual sharks and pikes. But in the majority of the Selachii it has already degenerated to the extent that the radii on one side of the fin-rod have been partly or entirely lost, and are retained only on the other (Fig. 337). We thus get the uniserial fin, which has been transmitted from the Selachii to the rest of the fishes (Fig. 338).
Gegenbaur has shown how the five-toed leg of the Amphibia, that has been inherited by the three classes of Amniotes, was evolved from the uniserial fish-fin.[32]
[32] The limb of the four higher classes of Vertebrates is now explained in the sense that the original fin-rod passes along its outer (ulnar or fibular) side, and ends in the fifth toe. It was formerly believed to go along the inner (radial or tibial) side, and end in the first toe, as Fig. 339 shows.) In the dipneust ancestors of the Amphibia the radii gradually atrophy, and are lost, for the most part, on the other side of the fin-rod as well (the lighter cartilages in Fig. 338). Only the four lowest radii (shaded in the illustration) are preserved; and these are the four inner toes of the foot (first to fourth). The little or fifth toe is developed from the lower end of the fin-rod. From the middle and upper part of the fin-rod was developed the long stem of the limb—the important radius and ulna (Fig. 339 r and u) and humerus (h) of the higher Vertebrates.
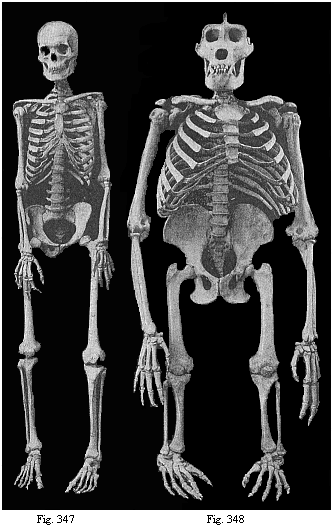
Fig. 347—Human skeleton. (Cf. Figure
326.)
Fig. 348—Skeleton of the giant gorilla. (Cf. Figure
209.)
In this way the five-toed foot of the Amphibia, which we first meet in the Carboniferous Stegocephala (Fig. 260), and which was inherited from them by the reptiles on one side and the mammals on the other, was formed by gradual degeneration and differentiation from the many-toed fish-fin (Fig. 341). The reduction of the radii to four was accompanied by a further differentiation of the fin-rod, its transverse segmentation into upper and lower halves, and the formation of the zone of the limb, which is composed originally of three limbs before and behind in the higher Vertebrates. The simple arch of the original shoulder-zone divides on each side into an upper (dorsal) piece, the shoulder-blade (scapula), and a lower (ventral) piece; the anterior part of the latter forms the primitive clavicle (procoracoideum), and the posterior part the coracoideum. In the same way the simple arch of the pelvic zone breaks up into an upper (dorsal) piece, the iliac-bone (os ilium), and a lower (ventral) piece; the anterior part of the latter forms the pubic bone (os pubis), and the posterior the ischial bone (os ischii).
There is also a complete agreement between the fore and hind limb in the stem or shaft. The first section of the stem is supported by a single strong bone—the humerus in the fore, the femur in the hind limb. The second section contains two bones: in front the radius (r) and ulna (u), behind the tibia and fibula. (Cf. the skeletons in Figs. 260, 265, 270, 278–282, and 348.) The succeeding numerous small bones of the wrist (carpus) and ankle (tarsus) are also similarly arranged in the fore and hind extremities, and so are the five bones of the middle-hand (metacarpus) and middle-foot (metatarsus). Finally, it is the same with the toes themselves, which have a similar characteristic composition from a series of bony pieces before and behind. We find a complete parallel in all the parts of the fore leg and the hind leg.
When we thus learn from comparative anatomy that the skeleton of the human limbs is composed of just the same bones, put together in the same way, as the skeleton in the four higher classes of Vertebrates, we may at once infer a common descent of them from a single stem-form. This stem-form was the earliest amphibian that had five toes on each foot. It is particularly the outer parts of the limbs that have been modified by adaptation to different conditions. We need only recall the immense variations they offer within the mammal class. We have the slender legs of the deer and the strong springing legs of the kangaroo, the climbing feet of the sloth and the digging feet of the mole, the fins of the whale and the wings of the bat. It will readily be granted that these organs of locomotion differ as much in regard to size, shape, and special function as can be conceived. Nevertheless, the bony skeleton is substantially the same in every case. In the different limbs we always find the same characteristic bones in essentially the same rigidly hereditary connection; this is as splendid a proof of the theory of evolution as comparative anatomy can discover in any organ of the body. It is true that the skeleton of the limbs of the various mammals undergoes many distortions and degenerations besides the special adaptations (Fig. 342). Thus we find the first finger or the thumb atrophied in the fore-foot (or hand) of the dog (II). It has entirely disappeared in the pig (III) and tapir (V). In the ruminants (such as the ox, IV) the second and fifth toes are also atrophied, and only the third and fourth are well developed (VI, 3). Nevertheless, all these different fore-feet, as well as the hand of the ape (Fig. 340) and of man (Fig. 341), were originally developed from a common pentadactyle stem-form. This is proved by the rudiments of the degenerated toes, and by the similarity of the arrangement of the wrist-bones in all the pentanomes (Fig. 342 a–p).
If we candidly compare the bony skeleton of the human arm and hand with that of the nearest anthropoid apes, we find an almost perfect identity. This is especially true of the chimpanzee. In regard to the proportions of the various parts, the lowest living races of men (the Veddahs of Ceylon, Fig. 344) are midway between the chimpanzee (Fig. 343) and the European (Fig. 345). More considerable are the differences in structure and the proportions of the various parts between the different genera of anthropoid apes (Figs. 278–282); and still greater is the morphological distance between these and the lowest apes (the Cynopitheca). Here, again, impartial and thorough anatomic comparison confirms the accuracy of Huxley’s pithecometra principle p. 171.
The complete unity of structure which is thus revealed by the comparative anatomy of the limbs is fully confirmed by their embryology. However different the extremities of the four-footed Craniotes may be in their adult state, they all develop from the same rudimentary structure. In every case the first trace of the limb in the embryo is a very simple protuberance that grows out of the side of the hyposoma. These simple structures develop directly into fins in the fishes and Dipneusts by differentiation of their cells. In the higher classes of Vertebrates each of the four takes the shape in its further growth of a leaf with a stalk, the inner half becoming narrower and thicker and the outer half broader and thinner. The inner half (the stalk of the leaf) then divides into two sections—the upper and lower parts of the limb. Afterwards four shallow indentations are formed at the free edge of the leaf, and gradually deepen; these are the intervals between the five toes (Fig. 174). The toes soon make their appearance. But at first all five toes, both of fore and hind feet, are connected by a thin membrane like a swimming-web; they remind us of the original shaping of the foot as a paddling fin. The further development of the limbs from this rudimentary structure takes place in the same way in all the Vertebrates according to the laws of heredity.
The embryonic development of the muscles, or active organs of locomotion, is not less interesting than that of the skeleton, or passive organs. But the comparative anatomy and ontogeny of the muscular system are much more difficult and inaccessible, and consequently have hitherto been less studied. We can therefore only draw some general phylogenetic conclusions therefrom.
It is incontestable that the musculature of the Vertebrates has been evolved from that of lower Invertebrates; and among these we have to consider especially the unarticulated Vermalia. They have a simple cutaneous muscular layer, developing from the mesoderm. This was afterwards replaced by a pair of internal lateral muscles, that developed from the middle wall of the cœlom-pouches; we still find the first rudiments of the muscles arising from the muscle-plate of these in the embryos of all the Vertebrates (cf. Figs. 124, 158–160, 222–224 mp). In the unarticulated stem-forms of the Chordonia, which we have called the Prochordonia, the two cœlom-pouches, and therefore also the muscle-plates of their walls, were not yet segmented. A great advance was made in the articulation of them, as we have followed it step by step in the Amphioxus (Figs. 124, 158). This segmentation of the muscles was the momentous historical process with which vertebration, and the development of the vertebrate stem, began. The articulation of the skeleton came after this segmentation of the muscular system, and the two entered into very close correlation.
The episomites or dorsal cœlom-pouches of the Acrania, Cyclostomes, and Selachii (Fig. 161 h) first develop from their inner or median wall (from the cell-layer that lies directly on the skeletal plate [sk] and the medullary tube [nr]) a strong muscle-plate (mp). By dorsal growth (w) it also reaches the external wall of the cœlom-pouches, and proceeds from the dorsal to the ventral wall. From these segmental muscle-plates, which are chiefly concerned in the segmentation of the Vertebrates, proceed the lateral muscles of the stem, as we find in the simplest form in the Amphioxus (Fig. 210). By the formation of a horizontal frontal septum they divide on each side into an upper and lower series of myotomes, dorsal and ventral lateral muscles. This is seen with typical regularity in the transverse section of the tail of a fish (Fig. 346). From these earlier lateral muscles of the trunk develop the greater part of the subsequent muscles of the trunk, and also the much later “muscular buds” of the limbs.[33]
[33] The ontogeny of the muscles is mostly cenogenetic. The greater part of the muscles of the head (or the visceral muscles) belong originally to the hyposoma of the vertebrate organism, and develop from the wall of the hyposomites or ventral cœlom-pouches. This also applies originally to the primary muscles of the limbs, as these too belong phylogenetically to the hyposoma. (Cf. Chapter XIV.)
The chief of the vegetal organs of the human frame, to the evolution of which we now turn our attention, is the alimentary canal. The gut is the oldest of all the organs of the metazoic body, and it leads us back to the earliest age of the formation of organs—to the first section of the Laurentian period. As we have already seen, the result of the first division of labour among the homogeneous cells of the earliest multicellular animal body was the formation of an alimentary cavity. The first duty and first need of every organism is self-preservation. This is met by the functions of the nutrition and the covering of the body. When, therefore, in the primitive globular Blastæa the homogeneous cells began to effect a division of labour, they had first to meet this twofold need. One half were converted into alimentary cells and enclosed a digestive cavity, the gut. The other half became covering cells, and formed an envelope round the alimentary tube and the whole body. Thus arose the primary germinal layers—the inner, alimentary, or vegetal layer, and the outer, covering, or animal layer. (Cf. pp. 214–17.)
When we try to construct an animal frame of the simplest conceivable type, that has some such primitive alimentary canal and the two primary layers constituting its wall, we inevitably come to the very remarkable embryonic form of the gastrula, which we have found with extraordinary persistence throughout the whole range of animals, with the exception of the unicellulars—in the Sponges, Cnidaria, Platodes, Vermalia, Molluscs, Articulates, Echinoderms, Tunicates, and Vertebrates. In all these stems the gastrula recurs in the same very simple form. It is certainly a remarkable fact that the gastrula is found in various animals as a larva-stage in their individual development, and that this gastrula, though much disguised by cenogenetic modifications, has everywhere essentially the same palingenetic structure (Figs. 30–35). The elaborate alimentary canal of the higher animals develops ontogenetically from the same simple primitive gut of the gastrula.
This gastræa theory is now accepted by nearly all zoologists. It was first supported and partly modified by Professor Ray-Lankester; he proposed three years afterwards (in his essay on the development of the Molluscs, 1875) to give the name of archenteron to the primitive gut and blastoporus to the primitive mouth.
Before we follow the development of the human alimentary canal in detail, it is necessary to say a word about the general features of its composition in the fully-developed man. The mature alimentary canal in man is constructed in all its main features like that of all the higher mammals, and particularly resembles that of the Catarrhines, the narrow-nosed apes of the Old World. The entrance into it, the mouth, is armed with thirty-two teeth, fixed in rows in the upper and lower jaws. As we have seen, our dentition is exactly the same as that of the Catarrhines, and differs from that of all other animals p. 257. Above the mouth-cavity is the double nasal cavity; they are separated by the palate-wall. But we saw that this separation is not there from the first, and that originally there is a common mouth-nasal cavity in the embryo; and this is only divided afterwards by the hard palate into two—the nasal cavity above and that of the mouth below (Fig. 311).
At the back the cavity of the mouth is half closed by the vertical curtain that we call the soft palate, in the middle of which is the uvula. A glance into a mirror with the mouth wide open will show its shape. The uvula is interesting because, besides man, it is only found in the ape. At each side of the soft palate are the tonsils. Through the curved opening that we find underneath the soft palate we penetrate into the gullet or pharynx behind the mouth-cavity. Into this opens on either side a narrow canal (the Eustachian tube), through which there is direct communication with the tympanic cavity of the ear (Fig. 320 e). The pharynx is continued in a long, narrow tube, the œsophagus ( sr). By this the food passes into the stomach when masticated and swallowed. Into the gullet also opens, right above, the trachea ( lr), that leads to the lungs. The entrance to it is covered by the epiglottis, over which the food slides. The cartilaginous epiglottis is found only in the mammals, and has developed from the fourth branchial arch of the fishes and amphibia. The lungs are found, in man and all the mammals, to the right and left in the pectoral cavity, with the heart between them. At the upper end of the trachea there is, under the epiglottis, a specially differentiated part, strengthened by a cartilaginous skeleton, the larynx. This important organ of human speech also develops from a part of the alimentary canal. In front of the larynx is the thyroid gland, which sometimes enlarges and forms goitre.
The œsophagus descends into the pectoral cavity along the vertebral column, behind the lungs and the heart, pierces the diaphragm, and enters the visceral cavity. The diaphragm is a membrano-muscular partition that completely separates the thoracic from the abdominal cavity in all the mammals (and these alone). This separation is not found in the beginning; there is at first a common breast-belly cavity, the cœloma or pleuro-peritoneal cavity. The diaphragm is formed later on as a muscular horizontal partition between the thoracic and abdominal cavities. It then completely separates the two cavities, and is only pierced by several organs that pass from the one to the other. One of the chief of these organs is the œsophagus. After this has passed through the diaphragm, it expands into the gastric sac in which digestion chiefly takes place. The stomach of the adult man (Fig. 349) is a long, somewhat oblique sac, expanding on the left into a blind sac, the fundus of the stomach ( b′), but narrowing on the right, and passing at the pylorus ( e) into the small intestine. At this point there is a valve, the pyloric valve ( d), between the two sections of the canal; it opens only when the pulpy food passes from the stomach into the intestine. In man and the higher Vertebrates the stomach itself is the chief organ of digestion, and is especially occupied with the solution of the food; this is not the case in many of the lower Vertebrates, which have no stomach, and discharge its function by a part of the gut farther on. The muscular wall of the stomach is comparatively thick; it has externally strong muscles that accomplish the digestive movements, and internally a large quantity of small glands, the peptic glands, which secrete the gastric juice.
Fig. 349—Human stomach and duodenum, longitudinal section. a cardiac (end of œsophagus), b fundus (blind sac of the left side), c pylorus-fold, d pylorus-valves, e pylorus-cavity, fgh duodenum, i entrance of the gall-duct and the pancreatic duct. (From Meyer.)
Next to the stomach comes the longest section of the alimentary canal, the middle gut or small intestine. Its chief function is to absorb the peptonised fluid mass of food, or the chyle, and it is subdivided into several sections, of which the first (next to the stomach) is called the duodenum (Fig. 349 fgh). It is a short, horseshoe-shaped loop of the gut. The largest glands of the alimentary canal open into it—the liver, the chief digestive gland, that secretes the gall, and the pancreas, which secretes the pancreatic juice. The two glands pour their secretions, the bile and pancreatic juice, close together into the duodenum ( i). The opening of the gall-duct is of particular phylogenetic importance, as it is the same in all the Vertebrates, and indicates the principal point of the hepatic or trunk-gut (Gegenbaur). The liver, phylogenetically older than the stomach, is a large gland, rich in blood, in the adult man, immediately under the diaphragm on the left side, and separated by it from the lungs. The pancreas lies a little further back and more to the left. The remaining part of the small intestine is so long that it has to coil itself in many folds in order to find room in the narrow space of the abdominal cavity. It is divided into the jejunum above and the ileum below. In the last section of it is the part of the small intestine at which in the embryo the yelk-sac opens into the gut. This long and thin intestine then passes into the large intestine, from which it is cut off by a special valve. Immediately behind this “Bauhin-valve” the first part of the large intestine forms a wide, pouch-like structure, the cæcum. The atrophied end of the cæcum is the famous rudimentary organ, the vermiform appendix. The large intestine ( colon) consists of three parts—an ascending part on the right, a transverse middle part, and a descending part on the left. The latter finally passes through an S-shaped bend into the last section of the alimentary canal, the rectum, which opens behind by the anus. Both the large and small intestines are equipped with numbers of small glands, which secrete mucous and other fluids.
Fig. 350—Median section of the head of a hare-embryo, one-fourth of an inch in length. (From Mihalcovics.) The deep mouth-cleft ( hp) is separated by the membrane of the throat ( rh) from the blind cavity of the head-gut ( kd). hz heart, ch chorda, hp the point at which the hypophysis develops from the mouth-cleft, vh ventricle of the cerebrum, v3 , third ventricle (intermediate brain), v4 fourth ventricle (hind brain), ck spinal canal.
For the greater part of its length the alimentary canal is attached to the inner dorsal surface of the abdominal cavity, or to the lower surface of the vertebral column. The fixing is accomplished by means of the thin membranous plate that we call the mesentery.
Although the fully-formed alimentary canal is thus a very elaborate organ, and although in detail it has a quantity of complex structural features into which we cannot enter here, nevertheless the whole complicated structure has been historically evolved from the very simple form of the primitive gut that we find in our gastræad-ancestors, and that every gastrula brings before us to-day. We have already pointed out (Chapter IX) how the epigastrula of the mammals (Fig. 67) can be reduced to the original type of the bell-gastrula, which is now preserved by the amphioxus alone (Fig. 35). Like the latter, the human gastrula and that of all other mammals must be regarded as the ontogenetic reproduction of the phylogenetic form that we call the Gastræa, in which the whole body is nothing but a double-walled gastric sac.
We already know from embryology the manner in which the gut develops in the embryo of man and the other mammals. From the gastrula is first formed the spherical embryonic vesicle filled with fluid ( gastrocystis, Fig. 106). In the dorsal wall of this the sole-shaped embryonic shield is developed, and on the under-side of this a shallow groove appears in the middle line, the first trace of the later, secondary alimentary tube. The gut-groove becomes deeper and deeper, and its edges bend towards each other, and finally form a tube.
As we have seen, this simple cylindrical gut-tube is at first completely closed before and behind in man and in the Vertebrates generally (Fig. 148); the permanent openings of the alimentary canal, the mouth and anus, are only formed later on, and from the outer skin. A mouth-pit appears in the skin in front (Fig. 350 hp), and this grows towards the blind fore-end of the cavity of the head-gut ( kd), and at length breaks into it. In the same way a shallow anus-pit is formed in the skin behind, which grows deeper and deeper, advances towards the blind hinder end of the pelvic gut, and at last connects with it. There is at first, both before and behind, a thin partition between the external cutaneous pit and the blind end of the gut—the throat-membrane in front and the anus-membrane behind; these disappear when the connection takes place.
Directly in front of the anus-opening the allantois develops from the hind gut; this is the important embryonic structure that forms into the placenta in the Placentals (including man). In this more advanced form the human alimentary canal (and that of all the other mammals) is a slightly bent, cylindrical tube, with an opening at each end, and two appendages growing from its lower wall: the anterior one is the umbilical vesicle or yelk-sac, and the posterior the allantois or urinary sac (Fig. 195).
The thin wall of this simple alimentary tube and its ventral appendages is found, on microscopic examination, to consist of two strata of cells. The inner stratum, lining the entire cavity, consists of larger and darker cells, and is the gut-gland layer. The outer stratum consists of smaller and lighter cells, and is the gut-fibre layer. The only exception is in the cavities of the mouth and anus, because these originate from the skin. The inner coat of the mouth-cavity is not provided by the gut-gland layer, but by the skin-sense layer; and its muscular substratum is provided, not by the gut-fibre, but the skin-fibre, layer. It is the same with the wall of the small anus-cavity.
If it is asked how these constituent layers of the primitive gut-wall are related to the various tissues and organs that we find afterwards in the fully-developed system, the answer is very simple. It can be put in a single sentence. The epithelium of the gut—that is to say, the internal soft stratum of cells that lines the cavity of the alimentary canal and all its appendages, and is immediately occupied with the processes of nutrition—is formed solely from the gut-gland layer; all other tissues and organs that belong to the alimentary canal and its appendages originate from the gut-fibre layer. From the latter is also developed the whole of the outer envelope of the gut and its appendages; the fibrous connective tissue and the smooth muscles that compose its muscular layer, the cartilages that support it (such as the cartilages of the larynx and the trachea), the blood-vessels and lymph-vessels that absorb the nutritive fluid from the intestines—in a word, all that there is in the alimentary system besides the epithelium of the gut. From the same layer we also get the whole of the mesentery, with all the organs embedded in it—the heart, the large blood-vessels of the body, etc.

Fig. 351—Scales or cutaneous teeth of a shark ( Centrophorus calceus). A three-pointed tooth rises obliquely on each of the quadrangular bony plates that lie in the corium. (From Gegenbaur.)
Let us now leave this original structure of the mammal gut for a moment, in order to compare it with the alimentary canal of the lower Vertebrates, and of those Invertebrates that we have recognised as man’s ancestors. We find, first of all, in the lowest Metazoa, the Gastræads, that the gut remains permanently in the very simple form in which we find it transitorily in the palingenetic gastrula of the other animals; it is thus in the Gastremaria ( Pemmatodiscus), the Physemaria ( Prophysema), the simplest Sponges ( Olynthus), the freshwater Polyps ( Hydra), and the ascula-embryos of many other Cœlenteria (Figs. 233–238). Even in the simplest forms of the Platodes, the Rhabdocœla (Fig. 240), the gut is still a simple straight tube, lined with the entoderm; but with the important difference that in this case its single opening, the primitive mouth ( m), has formed a muscular gullet ( sd) by invagination of the skin.
We have the same simple form in the gut of the lowest Vermalia (Gastrotricha, Fig. 242, Nematodes, Sagitta, etc.). But in these a second important opening of the gut has been formed at the opposite end to the mouth, the anus (Fig. 242 a).
We see a great advance in the structure of the vermalian gut in the remarkable Balanoglossus (Fig. 245), the sole survivor of the Enteropneust class. Here we have the first appearance of the division of the alimentary tube into two sections that characterises the Chordonia. The fore half, the head-gut ( cephalogaster), becomes the organ of respiration (branchial gut, Fig. 245 k); the hind half, the trunk-gut ( truncogaster), alone acts as digestive organ (hepatic gut, d). The differentiation of these two parts of the gut in the Enteropneust is just the same as in all the Tunicates and Vertebrates.
It is particularly interesting and instructive in this connection to compare the Enteropneusts with the Ascidia and the Amphioxus (Figs. 220, 210)—the remarkable animals that form the connecting link between the Invertebrates and the Vertebrates. In both forms the gut is of substantially the same construction; the anterior section forms the respiratory branchial gut, the posterior the digestive hepatic gut. In both it develops palingenetically from the primitive gut of the gastrula, and in both the hinder end of the medullary tube covers the primitive mouth to such an extent that the remarkable medullary intestinal duct is formed, the passing communication between the neural and intestinal tubes ( canalis neurentericus, Figs. 83, 85 ne). In the vicinity of the closed primitive mouth, possibly in its place, the later anus is developed. In the same way the mouth is a fresh formation in the Amphioxus and the Ascidia. It is the same with the human mouth and that of the Craniotes generally. The secondary formation of the mouth in the Chordonia is probably connected with the development of the gill-clefts which are formed in the gut-wall immediately behind the mouth. In this way the anterior section of the gut is converted into a respiratory organ. I have already pointed out that this modification is distinctive of the Vertebrates and Tunicates. The phylogenetic appearance of the gill-clefts indicates the commencement of a new epoch in the stem-history of the Vertebrates.
In the further ontogenetic development of the alimentary canal in the human embryo the appearance of the gill-clefts is the most important process. At a very early stage the gullet-wall joins with the external body-wall in the head of the human embryo, and this is followed by the formation of four clefts, which lead directly into the gullet from without, on the right and left sides of the neck, behind the mouth. These are the gill or gullet clefts, and the partitions that separate them are the gill or gullet-arches (Fig. 171). These are most interesting embryonic structures. They show us that all the higher Vertebrates reproduce in their earlier stages, in harmony with the biogenetic law, the process that had so important a part in the rise of the whole Chordonia-stem. This process was the differentiation of the gut into two sections—an anterior respiratory section, the branchial gut, that was restricted to breathing, and a posterior digestive section, the hepatic gut. As we find this highly characteristic differentiation of the gut into two different sections in all the Vertebrates and all the Tunicates, we may conclude that it was also found in their common ancestors, the Prochordonia—especially as even the Enteropneusts have it. (Cf. pp. 119, 151, 227, Figs. 210, 220, 245.) It is entirely wanting in all the other Invertebrates.
Fig. 353—Gut of a dog-embryo (shown in
Fig. 202, from Bischoff), seen from the ventral side. a
gill-arches (four pairs), b rudiments of pharynx and larynx, c
lungs, d stomach, f liver, g walls of the open yelk-sac
(into which the middle gut opens with a wide aperture), h rectum.
Fig. 354—The same gut seen from the right. a lungs,
b stomach, c liver, d yelk-sac, e rectum.)
There is at first only one pair of gill-clefts in the Amphioxus, as in the Ascidia and Enteropneusts; and the Copelata (Fig. 225) have only one pair throughout life. But the number presently increases in the former. In the Craniotes, however, it decreases still further. The Cyclostomes have six to eight pairs (Fig. 247); some of the Selachii six or seven pairs, most of the fishes only four or five pairs. In the embryo of man, and the higher Vertebrates generally, where they make an appearance at an early stage, only three or four pairs are developed. In the fishes they remain throughout life, and form an exit for the water taken in at the mouth (Figs. 249–251). But they are partly lost in the amphibia, and entirely in the higher Vertebrates. In these nothing is left but a relic of the first gill-cleft. This is formed into a part of the organ of hearing; from it are developed the external meatus, the tympanic cavity, and the Eustachian tube. We have already considered these remarkable structures, and need only point here to the interesting fact that our middle and external ear is a modified inheritance from the fishes. The branchial arches also, which separate the clefts, develop into very different parts. In the fishes they remain gill-arches, supporting the respiratory gill-leaves. It is the same with the lowest amphibia, but in the higher amphibia they undergo various modifications; and in the three higher classes of Vertebrates (including man) the hyoid bone and the ossicles of the ear develop from them. (Cf. p. 291.)
From the first gill-arch, from the inner surface of which the muscular tongue proceeds, we get the first structure of the maxillary skeleton—the upper and lower jaws, which surround the mouth and support the teeth. These important parts are wholly wanting in the two lowest classes of Vertebrates, the Acrania and Cyclostoma. They appear first in the earliest Selachii (Figs. 248–251), and have been transmitted from this stem-group of the Gnathostomes to the higher Vertebrates. Hence the original formation of the skeleton of the mouth can be traced to these primitive fishes, from which we have inherited it. The teeth are developed from the skin that clothes the jaws. As the whole mouth cavity originates from the outer integument (Fig. 350), the teeth also must come from it. As a fact, this is found to be the case on microscopic examination of the development and finer structure of the teeth. The scales of the fishes, especially of the shark type (Fig. 351), are in the same position as their teeth in this respect (Fig. 252). The osseous matter of the tooth (dentine) develops from the corium; its enamel covering is a secretion of the epidermis that covers the corium. It is the same with the cutaneous teeth or placoid scales of the Selachii. At first the whole of the mouth was armed with these cutaneous teeth in the Selachii and in the earliest amphibia. Afterwards the formation of them was restricted to the edges of the jaws.
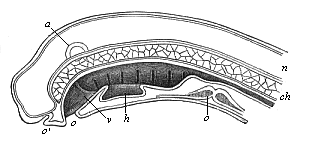
Fig. 355—Median section of the head of a Petromyzon-larva. (From Gegenbaur.) h hypobranchial groove (above it in the gullet we see the internal openings of the seven gill-clefts), v velum, o mouth, c heart, a auditory vesicle, n neural tube, ch chorda.
Hence our human teeth are, in relation to their original source, modified fish-scales. For the same reason we must regard the salivary glands, which open into the mouth, as epidermic glands, as they are formed, not from the glandular layer of the gut like the rest of the alimentary glands, but from the epidermis, from the horny plate of the outer germinal layer. Naturally, in harmony with this evolution of the mouth, the salivary glands belong genetically to one series with the sudoriferous, sebaceous, and mammary glands.
Thus the human alimentary canal is as simple as the primitive gut of the gastrula in its original structure. Later it resembles the gut of the earliest Vermalia (Gastrotricha). It then divides into two sections, a fore or branchial gut and a hind or hepatic gut, like the alimentary canal of the Balanoglossus, the Ascidia, and the Amphioxus. The formation of the jaws and the branchial arches changes it into a real fish-gut ( Selachii). But the branchial gut, the one reminiscence of our fish-ancestors, is afterwards atrophied as such. The parts of it that remain are converted into entirely different structures.
Fig. 356—Transverse section of the head of a Petromyzon-larva. (From Gegenbaur.) Beneath the pharynx ( d) we see the hypobranchial groove; above it the chorda and neural tube. A, B, C stages of constriction.
But, although the anterior section of our alimentary canal thus entirely loses its original character of branchial gut, it retains the physiological character of respiratory gut. We are now astonished to find that the permanent respiratory organ of the higher Vertebrates, the air-breathing lung, is developed from this first part of the alimentary canal. Our lungs, trachea, and larynx are formed from the ventral wall of the branchial gut. The whole of the respiratory apparatus, which occupies the greater part of the pectoral cavity in the adult man, is at first merely a small pair of vesicles or sacs, which grow out of the floor of the head-gut immediately behind the gills (Figs. 354 c, 147 l). These vesicles are found in all the Vertebrates except the two lowest classes, the Acrania and Cyclostomes. In the lower Vertebrates they do not develop into lungs, but into a large air-filled bladder, which occupies a good deal of the body-cavity and has a quite different purport. It serves, not for breathing, but to effect swimming movements up and down, and so is a sort of hydrostatic apparatus—the floating bladder of the fishes ( nectocystis, p. 233). However, the human lungs, and those of all air-breathing Vertebrates, develop from the same simple vesicular appendage of the head-gut that becomes the floating bladder in the fishes.
At first this bladder has no respiratory function, but merely acts as hydrostatic apparatus for the purpose of increasing or lessening the specific gravity of the body. The fishes, which have a fully-developed floating bladder, can press it together, and thus condense the air it contains. The air also escapes sometimes from the alimentary canal, through an air-duct that connects the floating bladder with the pharynx, and is ejected by the mouth. This lessens the size of the bladder, and so the fish becomes heavier and sinks. When it wishes to rise again, the bladder is expanded by relaxing the pressure. In many of the Crossopterygii the wall of the bladder is covered with bony plates, as in the Triassic Undina (Fig. 254).
This hydrostatic apparatus begins in the Dipneusts to change into a respiratory organ; the blood-vessels in the wall of the bladder now no longer merely secrete air themselves, but also take in fresh air through the air-duct. This process reaches its full development in the Amphibia. In these the floating bladder has turned into lungs, and the air-passage into a trachea. The lungs of the Amphibia have been transmitted to the three higher classes of Vertebrates. In the lowest Amphibia the lungs on either side are still very simple transparent sacs with thin walls, as in the common water-salamander, the Triton. It still entirely resembles the floating bladder of the fishes. It is true that the Amphibia have two lungs, right and left. But the floating bladder is also double in many of the fishes (such as the early Ganoids), and divides into right and left halves. On the other hand, the lung is single in Ceratodus (Fig. 257).
Fig. 357—Thoracic and abdominal viscera of a human embryo of twelve weeks. (From Kölliker.) The head is omitted. Ventral and pectoral walls are removed. The greater part of the body-cavity is taken up with the liver, from the middle part of which the cæcum and the vermiform appendix protrude. Above the diaphragm, in the middle, is the conical heart; to the right and left of it are the two small lungs.
In the human embryo and that of all the other Amniotes the lungs develop from the hind part of the ventral wall of the head-gut (Fig. 149). Immediately behind the single structure of the thyroid gland a median groove, the rudiment of the trachea, is detached from the gullet. From its hinder end a couple of vesicles develop—the simple tubular rudiments of the right and left lungs. They afterwards increase considerably in size, fill the greater part of the thoracic cavity, and take the heart between them. Even in the frogs we find that the simple sac has developed into a spongy body of peculiar froth-like tissue. The originally short connection of the pulmonary sacs with the head-gut extends into a long, thin tube. This is the wind-pipe (trachea); it opens into the gullet above, and divides below into two branches which go to the two lungs. In the wall of the trachea circular cartilages develop, and these keep it open. At its upper end, underneath its pharyngeal opening, the larynx is formed—the organ of voice and speech. The larynx is found at various stages of development in the Amphibia, and comparative anatomists are in a position to trace the progressive growth of this important organ from the rudimentary structure of the lower Amphibia up to the elaborate and delicate vocal apparatus that we have in the larynx of man and of the birds.
We must refer here to an interesting rudimentary organ of the respiratory gut, the thyroid gland, the large gland in front of the larynx, that lies below the “Adam’s apple,” and is often especially developed in the male sex. It has a certain function—not yet fully understood—in the nutrition of the body, and arises in the embryo by constriction from the lower wall of the pharynx. In many mining districts the thyroid gland is peculiarly liable to morbid enlargement, and then forms goitre, a growth that hangs at the front of the neck. But it is much more interesting phylogenetically. As Wilhelm Müller, of Jena, has shown, this rudimentary organ is the last relic of the hypobranchial groove, which we considered in a previous chapter, and which runs in the middle line of the gill-crate in the Ascidia and Amphioxus, and conveys food to the stomach. (Cf. p. 184,Fig. 246). We still find it in its original character in the larvæ of the Cyclostomes (Figs. 355, 356).
The second section of the alimentary canal, the trunk or hepatic gut, undergoes not less important modifications among our vertebrate ancestors than the first section. In tracing the further development of this digestive part of the gut, we find that most complex and elaborate organs originate from a very rudimentary original structure. For clearness we may divide the digestive gut into three sections: the fore gut (with œsophagus and stomach), the middle gut (duodenum, with liver, pancreas, jejunum, and ileum, and the hind gut (colon and rectum). Here again we find vesicular growths or appendages of the originally simple gut developing into a variety of organs. Two of these embryonic structures, the yelk-sac and allantois, are already known to us. The two large glands that open into the duodenum, the liver and pancreas, are growths from the middle and most important part of the trunk-gut.
Immediately behind the vesicular rudiments of the lungs comes the section of the alimentary canal that forms the stomach (Figs. 353 d, 354 b). This sac-shaped organ, which is chiefly responsible for the solution and digestion of the food, has not in the lower Vertebrates the great physiological importance and the complex character that it has in the higher. In the Acrania and Cyclostomes and the earlier fishes we can scarcely distinguish a real stomach; it is represented merely by the short piece from the branchial to the hepatic gut. In some of the other fishes also the stomach is only a very simple spindle-shaped enlargement at the beginning of the digestive section of the gut, running straight from front to back in the median plane of the body, underneath the vertebral column. In the mammals its first structure is just as rudimentary as it is permanently in the preceding. But its various parts soon begin to develop. As the left side of the spindle-shaped sac grows much more quickly than the right, and as it turns considerably on its axis at the same time, it soon comes to lie obliquely. The upper end is more to the left, and the lower end more to the right. The foremost end draws up into the longer and narrower canal of the œsophagus. Underneath this on the left the blind sac (fundus) of the stomach bulges out, and thus the later form gradually develops (Figs. 349, 184 e). The original longitudinal axis becomes oblique, sinking below to the left and rising to the right, and approaches nearer and nearer to a transverse position. In the outer layer of the stomach-wall the powerful muscles that accomplish the digestive movements develop from the gut-fibre layer. In the inner layer a number of small glandular tubes are formed from the gut-gland layer; these are the peptic glands that secrete the gastric juice. At the lower end of the gastric sac is developed the valve that separates it from the duodenum (the pylorus, Fig. 349 d).
Underneath the stomach there now develops the disproportionately long stretch of the small intestine. The development of this section is very simple, and consists essentially in an extremely rapid and considerable growth lengthways. It is at first very short, quite straight, and simple. But immediately behind the stomach we find at an early stage a horseshoe-shaped bend and loop of the gut, in connection with the severance of the alimentary canal from the yelk-sac and the development of the first mesentery. The thin delicate membrane that fastens this loop to the ventral side of the vertebral column, and fills the inner bend of the horseshoe formation, is the first rudiment of the mesentery (Fig. 147 g). We find at an early stage a considerable growth of the small intestine; it is thus forced to coil itself in a number of loops. The various sections that we have to distinguish in it are differentiated in a very simple way—the duodenum (next to the stomach), the succeeding long jejunum, and the last section of the small intestine, the ileum.
From the duodenum are developed the two large glands that we have already mentioned—the liver and pancreas. The liver appears first in the shape of two small sacs, that are found to the right and left immediately behind the stomach (Figs. 353 f, 354 c). In many of the lower Vertebrates they remain separate for a long time (in the Myxinoides throughout life), or are only imperfectly joined. In the higher Vertebrates they soon blend more or less completely to form a single large organ. The growth of the liver is very brisk at first. In the human embryo it grows so much in the second month of development that in the third it occupies by far the greater part of the body-cavity (Fig. 357). At first the two halves develop equally; afterwards the left falls far behind the right. In consequence of the unsymmetrical development and turning of the stomach and other abdominal viscera, the whole liver is now pushed to the right side. Although the liver does not afterwards grow so disproportionately, it is comparatively larger in the embryo at the end of pregnancy than in the adult. Its weight relatively to that of the whole body is 1 : 36 in the adult, and 1 : 18 in the embryo. Hence it is very important physiologically during embryonic life; it is chiefly concerned in the formation of blood, not so much in the secretion of bile.
Immediately behind the liver a second large visceral gland develops from the duodenum, the pancreas or sweetbread. It is wanting in most of the lowest classes of Vertebrates, and is first found in the fishes. This organ is also an outgrowth from the gut.
The last section of the alimentary canal, the large intestine, is at first in the embryo a very simple, short, and straight tube, which opens behind by the anus. It remains thus throughout life in the lower Vertebrates. But it grows considerably in the mammals, coils into various folds, and divides into two sections, the first and longer of which is the colon, and the second the rectum. At the beginning of the colon there is a valve (valvula Bauhini) that separates it from the small intestine. Immediately behind this there is a sac-like growth, which enlarges into the cæcum (Fig. 357 v). In the plant-eating mammals this is very large, but it is very small or completely atrophied in the flesh-eaters. In man, and most of the apes, only the first portion of the cæcum is wide; the blind end-part of it is very narrow, and seems later to be merely a useless appendage of the former. This “vermiform appendage” is very interesting as a rudimentary organ. The only significance of it in man is that not infrequently a cherry-stone or some other hard and indigestible matter penetrates into its narrow cavity, and by setting up inflammation and suppuration causes the death of otherwise sound men. Teleology has great difficulty in giving a rational explanation of, and attributing to a beneficent Providence, this dreaded appendicitis. In our plant-eating ancestors this rudimentary organ was much larger and had a useful function.
Finally, we have important appendages of the alimentary tube in the bladder and urethra, which belong to the alimentary system. These urinary organs, acting as reservoir and duct for the urine excreted by the kidneys, originate from the innermost part of the allantoic pedicle. In the Dipneusts and Amphibia, in which the allantoic sac first makes its appearance, it remains within the body-cavity, and functions entirely as bladder. But in all the Amniotes it grows far outside of the body-cavity of the embryo, and forms the large embryonic “primitive bladder,” from which the placenta develops in the higher mammals. This is lost at birth. But the long stalk or pedicle of the allantois remains, and forms with its upper part the middle vesico-umbilical ligament, a rudimentary organ that goes in the shape of a solid string from the vertex of the bladder to the navel. The lowest part of the allantoic pedicle (or the “urachus”) remains hollow, and forms the bladder. At first this opens into the last section of the gut in man as in the lower Vertebrates; thus there is a real cloaca, which takes off both urine and excrements. But among the mammals this cloaca is only permanent in the Monotremes, as it is in all the birds, reptiles, and amphibia. In all the other mammals (marsupials and placentals) a transverse partition is afterwards formed, and this separates the urogenital aperture in front from the anus-opening behind. (Cf. p. 249 and Chapter 29.)
The use that we have hitherto made of our biogenetic law will give the reader an idea how far we may trust its guidance in phylogenetic investigation. This differs considerably in the various systems of organs; the reason is that heredity and variability have a very different range in these systems. While some of them faithfully preserve the original palingenetic development inherited from earlier animal ancestors, others show little trace of this rigid heredity; they are rather disposed to follow new and divergent cenogenetic lines of development in consequence of adaptation. The organs of the first kind represent the conservative element in the multicellular state of the human frame, while the latter represent the progressive element. The course of historic development is a result of the correlation of the two tendencies, and they must be carefully distinguished.
There is perhaps no other system of organs in the human body in which this is more necessary than in that of which we are now going to consider the obscure development—the vascular system, or apparatus of circulation. If we were to draw our conclusions as to the original features in our earlier animal ancestors solely from the phenomena of the development of this system in the embryo of man and the other higher Vertebrates, we should be wholly misled. By a number of important embryonic adaptations, the chief of which is the formation of an extensive food-yelk, the original course of the development of the vascular system has been so much falsified and curtailed in the higher Vertebrates that little or nothing now remains in their embryology of some of the principal phylogenetic features. We should be quite unable to explain these if comparative anatomy and ontogeny did not come to our assistance.
The vascular system in man and all the Craniotes is an elaborate apparatus of cavities filled with juices or cell-containing fluids. These “vessels” (vascula) play an important part in the nutrition of the body. They partly conduct the nutritive red blood to the various parts of the body (blood-vessels); partly absorb from the gut the white chyle formed in digestion (chyle-vessels); and partly collect the used-up juices and convey them away from the tissues (lymphatic vessels). With the latter are connected the large cavities of the body, especially the body-cavity, or cœloma. The lymphatic vessels conduct both the colourless lymph and the white chyle into the venous part of the circulation. The lymphatic glands act as producers of new blood-cells, and with them is associated the spleen. The centre of movement for the circulation of the fluids is the heart, a strong muscular sac, which contracts regularly and is equipped with valves like a pump. This constant and steady circulation of the blood makes possible the complex metabolism of the higher animals.
But, however important the vascular system may be to the more advanced and larger and highly-differentiated animals, it is not at all so indispensable an element of animal life as is commonly supposed. The older science of medicine regarded the blood as the real source of life. Even in the still prevalent confused notions of heredity the blood plays the chief part. People speak generally of full blood, half blood, etc., and imagine that the hereditary transmission of certain characters “lies in the blood.” The incorrectness of these ideas is clearly seen from the fact that in the act of generation the blood of the parents is not directly transmitted to the offspring, nor does the embryo possess blood in its early stages. We have already seen that not only the differentiation of the four secondary germinal layers, but also the first structures of the principal organs in the embryo of all the Vertebrates, take place long before there is any trace of the vascular system—the heart and the blood. In accordance with this ontogenetic fact, we must regard the vascular system as one of the latest organs from the phylogenetic point of view; just as we have found the alimentary canal to be one of the earliest. In any case, the vascular system is much later than the alimentary.
Fig. 358—Red blood-cells of various
Vertebrates (equally magnified). 1. of man, 2. camel,
3. dove, 4. proteus, 5. water-salamander (Triton),
6. frog, 7. merlin (Cobitis), 8. lamprey
(Petromyzon). a surface-view, b edge-view. (From
Wagner.)
Fig. 359—Vascular tissues or endothelium
(vasalium). A capillary from the mesentery. a vascular cells,
b their nuclei.
The important nutritive fluid that circulates as blood and lymph in the elaborate canals of our vascular system is not a clear, simple fluid, but a very complex chemical juice with millions of cells floating in it. These blood-cells are just as important in the complicated life of the higher animal body as the circulation of money is to the commerce of a civilised community. Just as the citizens meet their needs most conveniently by means of a financial circulation, so the various tissue-cells, the microscopic citizens of the multicellular human body, have their food conveyed to them best by the circulating cells in the blood. These blood cells (hæmocytes) are of two kinds in man and all the other Craniotes—red cells (rhodocytes or erythrocytes) and colourless or lymph cells (leucocytes). The red colour of the blood is caused by the great accumulation of the former, the others circulate among them in much smaller quantity. When the colourless cells increase at the expense of the red we get anæmia (or chlorosis).
The lymph-cells (leucocytes), commonly called the “white corpuscles” of the blood, are phylogenetically older and more widely distributed in the animal world than the red. The great majority of the Invertebrates that have acquired an independent vascular system have only colourless lymph-cells in the circulating fluid. There is an exception in the Nemertines (Fig. 358) and some groups of Annelids. When we examine the colourless blood of a cray-fish or a snail (Fig. 358) under a high power of the microscope, we find in each drop numbers of mobile leucocytes, which behave just like independent Amoebæ (Fig. 17). Like these unicellular Protozoa, the colourless blood-cells creep slowly about, their unshapely plasma-body constantly changing its form, and stretching out finger-like processes first in one direction, then another. Like the Amoebæ, they take particles into their cell-body. On account of this feature these amoeboid plastids are called “eating cells” (phagocytes), and on account of their motions “travelling cells” (planocytes). It has been shown by the discoveries of the last few decades that these leucocytes are of the greatest physiological and pathological consequence to the organism. They can absorb either solid or dissolved particles from the wall of the gut, and convey them to the blood in the chyle; they can absorb and remove unusable matter from the tissues. When they pass in large quantities through the fine pores of the capillaries and accumulate at irritated spots, they cause inflammation. They can consume and destroy bacteria, the dreaded vehicles of infectious diseases; but they can also transport these injurious Monera to fresh regions, and so extend the sphere of infection. It is probable that the sensitive and travelling leucocytes of our invertebrate ancestors have powerfully co-operated for millions of years in the phylogenesis of the advancing animal organisation.
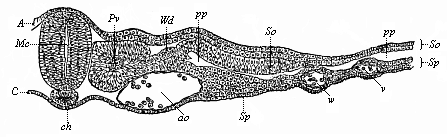
Fig. 360—Transverse section of the trunk of a chick-embryo, forty-five hours old. (From Balfour.) A ectoderm (horny-plate), Mc medullary tube, ch chorda, C entoderm (gut-gland layer), Pv primitive segment (episomite), Wd prorenal duct, pp cœloma (secondary body-cavity). So skin-fibre layer, Sp gut-fibre layer, v blood-vessels in latter, ao primitive aortas, containing red blood-cells.
The red blood-cells have a much more restricted sphere of distribution and activity. But they also are very important in connection with certain functions of the craniote-organism, especially the exchange of gases or respiration. The cells of the dark red, carbonised or venous, blood, which have absorbed carbonic acid from the animal tissues, give this off in the respiratory organs; they receive instead of it fresh oxygen, and thus bring about the bright red colour that distinguishes oxydised or arterial blood. The red colouring matter of the blood (hæmoglobin) is regularly distributed in the pores of their protoplasm. The red cells of most of the Vertebrates are elliptical flat disks, and enclose a nucleus of the same shape; they differ a good deal in size (Fig. 358). The mammals are distinguished from the other Vertebrates by the circular form of their biconcave red cells and by the absence of a nucleus (Fig. 1); only a few genera still have the elliptic form inherited from the reptiles (Fig. 2). In the embryos of the mammals the red cells have a nucleus and the power of increasing by cleavage (Fig. 10).
The origin of the blood-cells and vessels in the embryo, and their relation to the germinal layers and tissues, are among the most difficult problems of ontogeny—those obscure questions on which the most divergent opinions are still advanced by the most competent scientists. In general, it is certain that the greater part of the cells that compose the vessels and their contents come from the mesoderm—in fact, from the gut-fibre layer; it was on this account that Baer gave the name of “vascular layer” to this visceral layer of the coeloma. But other important observers say that a part of these cells come from other germinal layers, especially from the gut-gland layer. It seems to be true that blood-cells may be formed from the cells of the entoderm before the development of the mesoderm. If we examine sections of chickens, the earliest and most familiar subjects of embryology, we find at an early stage the “primitive-aortas” we have already described (Fig. 360 ao) in the ventral angle between the episoma (Pv) and hyposoma (Sp). The thin wall of these first vessels of the amniote embryo consists of flat cells (endothelia or vascular epithelia); the fluid within already contains numbers of red blood-cells; both have been developed from the gut-fibre layer. It is the same with the vessels of the germinative area (Fig. 361 v), which lie on the entodermic membrane of the yelk-sac (c). These features are seen still more clearly in the transverse section of the duck-embryo in Fig. 152.In this we see clearly how a number of stellate cells proceed from the “vascular layer” and spread in all directions in the “primary body-cavity”—i.e. in the spaces between the germinal layers. A part of these travelling cells come together and line the wall of the larger spaces, and thus form the first vessels; others enter into the cavity, live in the fluid that fills it, and multiply by cleavage—the first blood-cells.
But, besides these mesodermic cells of the “vascular layer” proper, other travelling cells, of which the origin and purport are still obscure, take part in the formation of blood in the meroblastic Vertebrates (especially fishes). The chief of these are those that Ruckert has most aptly denominated “merocytes.” These “eating yelk-cells” are found in large numbers in the food-yelk of the Selachii, especially in the yelk-wall—the border zone of the germinal disk in which the embryonic vascular net is first developed. The nuclei of the merocytes become ten times as large as the ordinary cell-nucleus, and are distinguished by their strong capacity for taking colour, or their special richness in chromatin. Their protoplasmic body resembles the stellate cells of osseous tissue (astrocytes), and behaves just like a rhizopod (such as Gromia); it sends out numbers of stellate processes all round, which ramify and stretch into the surrounding food-yelk. These variable and very mobile processes, the pseudopodia of the merocytes, serve both for locomotion and for getting food; as in the real rhizopods, they surround the solid particles of food (granules and plates of yelk), and accumulate round their nucleus the food they have received and digested. Hence we may regard them both as eating-cells (phagocytes) and travelling-cells (planocytes). Their lively nucleus divides quickly and often repeatedly, so that a number of new nuclei are formed in a short time; as each fresh nucleus surrounds itself with a mantle of protoplasm, it provides a new cell for the construction of the embryo. Their origin is still much disputed.
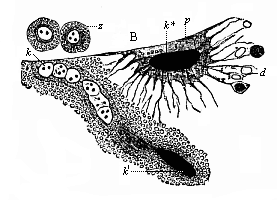
Fig. 361—Merocytes of a shark-embryo, rhizopod-like yelk-cells underneath the embryonic cavity (B). (From Ruckert.) z two embryonic cells, k nuclei of the merocytes, which wander about in the yelk and eat small yelk-plates (d), k smaller, more superficial, lighter nuclei, k′ a deeper nucleus, in the act of cleavage, k* chromatin-filled border-nucleus, freed from the surrounding yelk in order to show the numerous pseudopodia of the protoplasmic cell-body.
Half of the twelve stems of the animal world have no blood-vessels. They make their first appearance in the Vermalia. Their earliest source is the primary body-cavity, the simple space between the two primary germinal layers, which is either a relic of the segmentation-cavity, or is a subsequent formation. Amoeboid planocytes, which migrate from the entoderm and reach this fluid-filled primary cavity, live and multiply there, and form the first colourless blood-cells. We find the vascular system in this very simple form to-day in the Bryozoa, Rotatoria, Nematoda, and other lower Vermalia.
The first step in the improvement of this primitive vascular system is the formation of larger canals or blood-conducting tubes. The spaces filled with blood, the relics of the primary body-cavity, receive a special wall. “Blood-vessels” of this kind (in the narrower sense) are found among the higher worms in various forms, sometimes very simple, at other times very complex. The form that was probably the incipient structure of the elaborate vascular system of the Vertebrates (and of the Articulates) is found in two primordial principal vessels—a dorsal vessel in the middle line of the dorsal wall of the gut, and a ventral vessel that runs from front to rear in the middle line of its ventral wall. From the dorsal vessel is evolved the aorta (or principal artery), from the ventral vessel the principal or subintestinal vein. The two vessels are connected in front and behind by a loop that runs round the gut. The blood contained in the two tubes is propelled by their peristaltic contractions.
Fig. 362—Vascular system of an Annelid (Sænuris), foremost section. d dorsal vessel, v ventral vessel, c transverse connection of two (enlarged in shape of heart). The arrows indicate the direction of the flow of blood. (From Gegenbaur.
The earliest Vermalia in which we first find this independent vascular system are the Nemertina (Fig. 244). As a rule, they have three parallel longitudinal vessels connected by loops, a single dorsal vessel above the gut and a pair of lateral vessels to the right and left. In some of the Nemertina the blood is already coloured, and the red colouring matter is real hæmoglobin, connected with elliptical discoid cells, as in the Vertebrates. The further evolution of this rudimentary vascular system can be gathered from the class of the Annelids in which we find it at various stages of development. First, a number of transverse connections are formed between the dorsal and ventral vessels, which pass round the gut ring-wise (Fig. 362). Other vessels grow into the body-wall and ramify in order to convey blood to it. In addition to the two large vessels of the middle plane there are often two lateral vessels, one to the right and one to the left; as, for instance, in the leech. There are four of these parallel longitudinal vessels in the Enteropneusts (Balanoglossus, Fig. 245). In these important Vermalia the foremost section of the gut has already been converted into a gill-crate, and the vascular arches that rise in the wall of this from the ventral to the dorsal vessel have become branchial vessels.
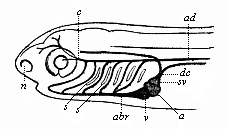
Fig. 363—Head of a fish-embryo, with rudimentary vascular system, from the left. dc Cuvier’s duct (juncture of the anterior and posterior principal veins), sv venous sinus (enlarged end of Cuvier’s duct), a auricle, v ventricle, abr trunk of branchial artery, s gill-clefts (arterial arches between), ad aorta, c carotid artery, n nasal pit. (From Gegenbaur.
We have a further important advance in the Tunicates, which we have recognised as the nearest blood-relatives of our early vertebrate ancestors. Here we find for the first time a real heart—i.e. a central organ of circulation, driving the blood into the vessels by the regular contractions of its muscular wall, it is of a very rudimentary character, a spindle-shaped tube, passing at both ends into a principal vessel (Fig. 221). By its original position behind the gill-crate, on ventral side of the Tunicates (sometimes more, sometimes less, forward), the head shows clearly that it has been formed by the local enlargement of a section of the ventral vessel. We have already noticed the remarkable alternation of the direction of the blood stream, the heart driving it first from one end, then from the other p. 190. This is very instructive, because in most of the worms (even the Enteropneust) the blood in the dorsal vessel travels from back to front, but in the Vertebrates in the opposite direction. As the Ascidia-heart alternates steadily from one direction to the other, it shows us permanently, in a sense, the phylogenetic transition from the earlier forward direction of the dorsal current (in the worms) to the new backward direction (in the Vertebrates).
As the new direction became permanent in the earlier Prochordonia, which gave rise to the Vertebrate stem, the two vessels that proceed from either end of the tubular heart acquired a fixed function. The foremost section of the ventral vessel henceforth always conveys blood from the heart, and so acts as an artery; the hind section of the same vessel brings the blood from the body to the heart, and so becomes a vein. In view of their relation to the two sections of the gut, we may call the latter the intestinal vein and the former the branchial artery. The blood contained in both vessels, and also in the heart, is venous or carbonised blood—i.e. rich in carbonic acid; on the other hand, the blood that passes from the gills into the dorsal vessel is provided with fresh oxygen—arterial or oxydised blood. The finest branches of the arteries and veins pass into each other in the tissues by means of a network of very fine, ventral, hair-like vessels, or capillaries (Fig. 359).
Fig. 364—The five arterial arches of the
Craniotes (1–5) in their original disposition. a
arterial cone or bulb, a″ aorta-trunk, c carotid artery
(foremost continuation of the roots of the aorta). (From Rathke.)
Fig. 365—The five arterial arches of the birds; the lighter parts
of the structure disappear; only the shaded parts remain. Letters as in Fig.
364. s subclavian arteries, p pulmonary artery, p′
branches of same, c′ outer carotid, c″ inner carotid.
(From Rathke.)
Fig. 366—The five arterial arches of
mammals; letters as in Fig. 365. v vertebral artery, b
Botall’s duct (open in the embryo, closed afterwards). (From
Rathke.)
When we turn from the Tunicates to the closely-related Amphioxus we are astonished at first to find an apparent retrogression in the formation of the vascular system. As we have seen, the Amphioxus has no real heart; its colourless blood is driven along in its vascular system by the principal vessel itself, which contracts regularly in its whole length (cf. Fig. 210). A dorsal vessel that lies above the gut (aorta) receives the arterial blood from the gills and drives it into the body. Returning from here, the venous blood gathers in a ventral vessel under the gut (intestinal vein), and goes back to the gills. A number of branchial vascular arches, which effect respiration and rise in the wall of the branchial gut from belly to back, absorb oxygen from the water and give off carbonic acid; they connect the ventral with the dorsal vessel. As the same section of the ventral vessel, which also forms the heart in the Craniotes, has developed in the Ascidia into a simple tubular heart, we may regard the absence of this in the Amphioxus as a result of degeneration, a return in this case to the earlier form of the vascular system, as we find it in many of the worms. We may assume that the Acrania that really belong to our ancestral series did not share this retrogression, but inherited the one-chambered heart of the Prochordonia, and transmitted it directly to the earliest Craniotes (cf. the ideal Primitive Vertebrate, Prospondylus, Figs. 98–102).
The further phylogenetic evolution of the vascular system is revealed to us by the comparative anatomy of the Craniotes. At the lowest stage of this group, in the Cyclostomes, we find for the first time the differentiation of the vasorium into two sections: a system of blood-vessels proper, which convey the red blood about the body, and a system of lymphatic vessels, which absorb the colourless lymph from the tissues and convey it to the blood. The lymphatics that absorb from the gut and pour into the blood-stream the milky food-fluid formed by digestion are distinguished by the special name of “chyle-vessels.” While the chyle is white on account of its high proportion of fatty particles, the lymph proper is colourless. Both chyle and lymph contain the colourless amœboid cells (leucocytes, Fig. 12) that we also find distributed in the blood as colourless blood-cells (or “white corpuscles”); but the blood also contains a much larger quantity of red cells, and these give its characteristic colour to the blood of the Craniotes (rhodocytes, Fig. 358). The distinction between lymph, chyle, and blood-vessels which is found in all the Craniotes may be regarded as an outcome of division of labour between various sections of our originally simple vascular system. In the Gnathostomes the spleen makes its first appearance, an organ rich in blood, the chief function of which is the extensive formation of new colourless and red cells. It is not found in the Acrania and Cyclostomes, or any of the Invertebrates. It has been transmitted from the earliest fishes to all the Craniotes.
Figs. 367–70—Metamorphosis of the five arterial arches in the human embryo (diagram from Rathke). la arterial cone, 1, 2, 3, 4, 5 first to fifth pair of arteries, ad trunk of aorta, aw roots of aorta. In Fig. 367 only three, in Fig. 368 all five, of the aortic arches are given (the dotted ones only are developed). In Fig. 369 the first two pairs have disappeared again. In Fig. 370 the permanent trunks of the artery are shown; the dotted parts disappear, s subclavian artery, v vertebral, ax axillary, c carotid (c′ outer, c″ inner carotid), p pulmonary.
The heart also, the central organ of circulation in all the Craniotes, shows an advance in structure in the Cyclostomes. The simple, spindle-shaped heart-tube, found in the same form in the embryo of all the Craniotes, is divided into two sections or chambers in the Cyclostomes, and these are separated by a pair of valves. The hind section, the auricle, receives the venous blood from the body and passes it on to the anterior section, the ventricle. From this it is driven through the trunk of the branchial artery (the foremost section of the ventral vessel or principal vein) into the gills.
In the Selachii an arterial cone is developed from the foremost end of the ventricle, as a special division, cut off by valves. It passes into the enlarged base of the trunk of the branchial artery (Fig. 363 abr). On each side 5–7 arteries proceed from it. These rise between the gill-clefts (s) on the gill-arches, surround the gullet, and unite above into a common trunk-aorta, the continuation of which over the gut corresponds to the dorsal vessel of the worms. As the curved arteries on the gill-arches spread into a network of respiratory capillaries, they contain venous blood in their lower part (as arches of the branchial artery) and arterial blood in the upper part (as arches of the aorta). The junctures of the various aortic arches on the right and left are called the roots of the aorta. Of an originally large number of aortic arches there remain at first six, then (owing to degeneration of the fifth arch) only five, pairs; and from these five pairs (Fig. 364) the chief parts of the arterial system develop in all the higher Vertebrates.
The appearance of the lungs and the atmospheric respiration connected therewith, which we first meet in the Dipneusts, is the next important step in vascular evolution. In the Dipneusts the auricle of the heart is divided by an incomplete partition into two halves. Only the right auricle now receives the venous blood from the veins of the body. The left auricle receives the arterial blood from the pulmonary veins. The two auricles have a common opening into the simple ventricle, where the two kinds of blood mix, and are driven through the arterial cone or bulb into the arterial arches. From the last arterial arches the pulmonary arteries arise (Fig. 365 p). These force a part of the mixed blood into the lungs, the other part of it going through the aorta into the body.
Fig. 371—Heart of a rabbit-embryo, from
behind. a vitelline veins, b auricles of the heart, c
atrium, d ventricle, e arterial bulb, f base of the three
pairs of arterial arches. (From Bischoff.)
Fig. 372—Heart
of the same embryo (Fig. 371), from the front. v vitelline veins,
a auricle, ca auricular canal, l left ventricle, r
right ventricle, ta arterial bulb. (From Bischoff.)
From the Dipneusts upwards we now trace a progressive development of the vascular system, which ends finally with the loss of branchial respiration and a complete separation of the two halves of the circulation. In the Amphibia the partition between the two auricles is complete. In their earlier stages, as tadpoles (Fig. 262), they have still the branchial respiration and the circulation of the fishes, and their heart contains venous blood alone. Afterwards the lungs and pulmonary vessels are developed, and henceforth the ventricle of the heart contains mixed blood. In the reptiles the ventricle and its arterial cone begin to divide into two halves by a longitudinal partition, and this partition becomes complete in the higher reptiles and birds on the one hand, and the stem-forms of the mammals on the other. Henceforth, the right half of the heart contains only venous, and the left half only arterial, blood, as we find in all birds and mammals. The right auricle receives its carbonised or venous blood from the veins of the body, and the right ventricle drives it through the pulmonary arteries into the lungs. From here the blood returns, as oxydised or arterial blood, through the pulmonary veins to the left auricle, and is forced by the left ventricle into the arteries of the body. Between the pulmonary arteries and veins is the capillary system of the small or pulmonary circulation. Between the body-arteries and veins is the capillary system of the large or body-circulation. It is only in the two highest classes of Vertebrates—the birds and mammals—that we find a complete division of the circulations. Moreover, this complete separation has been developed quite independently in the two classes, as the dissimilar formation of the aortas shows of itself. In the birds the right half of the fourth arterial arch has become the permanent arch (Fig. 365). In the mammals this has been developed from the left half of the same fourth arch (Fig. 366).
Fig. 373—Heart and head of a dog-embryo,
from the front. a fore brain, b eyes, c middle brain,
d primitive lower jaw, e primitive upper jaw, f
gill-arches, g right auricle, h left auricle, i left
ventricle, k right ventricle. (From Bischoff.)
Fig.
374—Heart of the same dog-embryo, from behind. a
inosculation of the vitelline veins, b left auricle, c right
auricle, d auricle, e auricular canal, f left ventricle,
g right ventricle, h arterial bulb. (From
Bischoff.)
If we compare the fully-developed arterial system of the various classes of Craniotes, it shows a good deal of variety, yet it always proceeds from the same fundamental type. Its development is just the same in man as in the other mammals; in particular, the modification of the six pairs of arterial arches is the same in both (Figs. 367–370). At first there is only a single pair of arches, which lie on the inner surface of the first pair of gill-arches. Behind this there then develop a second and third pair of arches (lying on the inner side of the second and third gill-arches, Fig. 367). Finally, we get a fourth, fifth, and sixth pair. Of the six primitive arterial arches of the Amniotes three soon pass away (the first, second, and fifth); of the remaining three, the third gives the carotids, the fourth the aortas, and the sixth (number 5 in Figs. 364 and 368) the pulmonary arteries.
Fig.
375—Heart of a human embryo, four weeks old; 1. front
view, 2. back view, 3. opened, and upper half of the atrium
removed. a′ left auricle, a″ right auricle,
v′ left ventricle, v″ right ventricle, ao
arterial bulb, c superior vena cava (cd right, cs left),
s rudiment of the interventricular wall. (From Kölliker.)
Fig. 376—Heart of a human embryo, six weeks old, front view.
r right ventricle, t left ventricle, s furrow between
ventricles, ta arterial bulb, af furrow on its surface; to right
and left are the two large auricles. (From Ecker.)
Fig.
377—Heart of a human embryo, eight weeks old, back view.
a′ left auricle, a″ right auricle, v′
left ventricle, v″ right ventricle, cd right superior vena
cava, ci inferior vena cava. (From Kölliker.)
The human heart also develops in just the same way as that of the other mammals (Fig. 378). We have already seen the first rudiments of its embryology, which in the main corresponds to its phylogeny (Figs. 201, 202). We saw that the palingenetic form of the heart is a spindle-shaped thickening of the gut-fibre layer in the ventral wall of the head-gut. The structure is then hollowed out, forms a simple tube, detaches from its place of origin, and henceforth lies freely in the cardiac cavity. Presently the tube bends into the shape of an S, and turns spirally on an imaginary axis in such a way that the hind part comes to lie on the dorsal surface of the fore part. The united vitelline veins open into the posterior end. From the anterior end spring the aortic arches.
Fig. 378—Heart of the adult man, fully developed, front view, natural position. a right auricle (underneath it the right ventricle), b left auricle (under it the left ventricle), C superior vena cava, V pulmonary veins, P pulmonary artery, d Botalli’s duct, A aorta. (From Meyer.)
This first structure of the human heart, enclosing a very simple cavity, corresponds to the tunicate-heart, and is a reproduction of that of the Prochordonia, but it now divides into two, and subsequently into three, compartments; this reminds us for a time of the heart of the Cyclostomes and fishes. The spiral turning and bending of the heart increases, and at the same time two transverse constrictions appear, dividing it externally into three sections (Figs. 371, 372). The foremost section, which is turned towards the ventral side, and from which the aortic arches rise, reproduces the arterial bulb of the Selachii. The middle section is a simple ventricle, and the hindmost, the section turned towards the dorsal side, into which the vitelline veins inosculate, is a simple auricle (or atrium). The latter forms, like the simple atrium of the fish-heart, a pair of lateral dilatations, the auricles (Fig. 371 b); and the constriction between the atrium and ventricle is called the auricular canal (Fig. 372 ca). The heart of the human embryo is now a complete fish-heart.
In perfect harmony with its phylogeny, the embryonic development of the human heart shows a gradual transition from the fish-heart, through the amphibian and reptile, to the mammal form, The most important point in the transition is the formation of a longitudinal partition—incomplete at first, but afterwards complete—which separates all three divisions of the heart into right (venous) and left (arterial) halves (cf. Figs. 373–378). The atrium is separated into a right and left half, each of which absorbs the corresponding auricle; into the right auricle open the body-veins (upper and lower vena cava, Figs. 375 c, 377 c); the left auricle receives the pulmonary veins. In the same way a superficial interventricular furrow is soon seen in the ventricle (Fig. 376 s). This is the external sign of the internal partition by which the ventricle is divided into two—a right venous and left arterial ventricle. Finally a longitudinal partition is formed in the third section of the primitive fish-like heart, the arterial bulb, externally indicated by a longitudinal furrow (Fig. 376 af). The cavity of the bulb is divided into two lateral halves, the pulmonary-artery bulb, that opens into the right ventricle, and the aorta-bulb, that opens into the left ventricle. When all the partitions are complete, the small (pulmonary) circulation is distinguished from the large (body) circulation; the motive centre of the former is the right half, and that of the latter the left half, of the heart.
Fig. 379—Transverse section of the back of the head of a chick-embryo, forty hours old. (From Kölliker.) m medulla oblongata, ph pharyngeal cavity (head-gut), h horny plate, h′ thicker part of it, from which the auscultory pits afterwards develop, hp skin-fibre plate, hh cervical cavity (head-cœlom or cardiocœl), hzp cardiac plate (the outermost mesodermic wall of the heart), connected by the ventral mesocardium (uhg) with the gut-fibre layer or visceral cœlom-layer (dfp*prime;), Ent entoderm, ihh inner (entodermic?) wall of the heart; the two endothelial cardiac tubes are still separated by the cenogenetic septum (s) of the Amniotes, g vessels.
The heart of all the Vertebrates belongs originally to the hyposoma of the head, and we accordingly find it in the embryo of man and all the other Amniotes right in front on the under-side of the head; just as in the fishes it remains permanently in front of the gullet. It afterwards descends into the trunk, with the advance in the development of the neck and breast, and at last reaches the breast, between the two lungs. At first it lies symmetrically in the middle plane of the body, so that its long axis corresponds with that of the body. In most of the mammals it remains permanently in this position. But in the apes the axis begins to be oblique, and the apex of the heart to move towards the left side. The displacement is greatest in the anthropoid apes—chimpanzee, gorilla, and orang—which resemble man in this.
As the heart of all Vertebrates is originally, in the light of phylogeny, only a local enlargement of the middle principal vein, it is in perfect accord with the biogenetic law that its first structure in the embryo is a simple spindle-shaped tube in the ventral wall of the head-gut. A thin membrane, standing vertically in the middle plane, the mesocardium, connects the ventral wall of the head-gut with the lower head-wall. As the cardiac tube extends and detaches from the gut-wall, it divides the mesocardium into an upper (dorsal) and lower (ventral) plate (usually called the mesocardium anterius and posterius in man, Fig. 379 uhg). The mesocardium divides two lateral cavities, Remak’s “neck-cavities” (Fig. 379 hh). These cavities afterwards join and form the simple pericardial cavity, and are therefore called by Kölliker the “primitive pericardial cavities.”
The double cervical cavity of the Amniotes is very interesting, both from the anatomical and the evolutionary point of view; it corresponds to a part of the hyposomites of the head of the lower Vertebrates—that part of the ventral cœlom-pouches which comes next to Van Wijhe’s “visceral cavities” below. Each of the cavities still communicates freely behind with the two cœlom-pouches of the trunk; and, just as these afterwards coalesce into a simple body-cavity (the ventral mesentery disappearing), we find the same thing happening in the head. This simple primary pericardial cavity has been well called by Gegenbaur the “head-cœloma,” and by Hertwig the “pericardial breast-cavity.” As it now encloses the heart, it may also be called cardiocœl.
Fig. 380—Frontal section of a human embryo, one-twelfth of an inch long in the neck; “invented” by Wilhelm His. Seen from ventral side. mb mouth-fissure, surrounded by the branchial processes, ab bulbus of aorta, hm middle part of ventricle, hl left lateral part of same, ho auricle, d diaphragm, vc superior vena cava, vu umbilical vein, vo vitelline space, lb liver, lg hepatic duct.
The cardiocœl, or head-cœlom, is often disproportionately large in the Amniotes, the simple cardiac tube growing considerably and lying in several folds. This causes the ventral wall of the amniote embryo, between the head and the navel, to be pushed outwards as in rupture (cf. Fig. 180 h). A transverse fold of the ventral wall, which receives all the vein-trunks that open into the heart, grows up from below between the pericardium and the stomach, and forms a transverse partition, which is the first structure of the primary diaphragm (Fig. 380 d). This important muscular partition, which completely separates the thoracic and abdominal cavities in the mammals alone, is still very imperfect here; the two cavities still communicate for a time by two narrow canals. These canals, which belong to the dorsal part of the head-cœlom, and which we may call briefly pleural ducts, receive the two pulmonary sacs, which develop from the hind end of the ventral wall of the head-gut; they thus become the two pleural cavities.
The diaphragm makes its first appearance in the class of the Amphibia (in the salamanders) as an insignificant muscular transverse fold of the ventral wall, which rises from the fore end of the transverse abdominal muscle, and grows between the pericardium and the liver. In the reptiles (tortoises and crocodiles) a later dorsal part is joined to this earlier ventral part of the rudimentary diaphragm, a pair of subvertebral muscles rising from the vertebral column and being added as “columns” to the transverse partition. But it was probably in the Permian sauro-mammals that the two originally separate parts were united, and the diaphragm became a complete partition between the thoracic and abdominal cavities in the mammals; as it considerably enlarges the chest-cavity when it contracts, it becomes an important respiratory muscle. The ontogeny of the diaphragm in man and the other mammals reproduces this phylogenetic process to-day, in accordance with the biogenetic law; in all the mammals the diaphragm is formed by the secondary conjunction of the two originally separate structures, the earlier ventral part and the later dorsal part.
Sometimes the blending of the two diaphragmatic structures, and consequently the severance of the one pleural duct from the abdominal cavity, is not completed in man. This leads to a diaphragmatic rupture (hernia diaphragmatica). The two cavities then remain in communication by an open pleural duct, and loops of the intestine may penetrate by this “rupture opening” into the chest-cavity. This is one of those fatal mis-growths that show the great part that blind chance has in organic development.
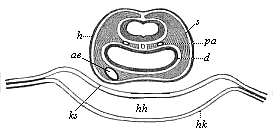
Fig. 381—Transverse section of the head of a chick-embryo, thirty-six hours old. Underneath the medullary tube the two primitive aortas (pa) can be seen in the head-plates (s) at each side of the chorda. Underneath the gullet (d) we see the aorta-end of the heart (ae), hh cervical cavity or head cœlom, hk top of heart, ks head-sheath, amniotic fold, h horny plate. (From Remak.
Thus the thoracic cavity of the mammals, with its important contents, the heart and lungs, belongs originally to the head-part of the vertebrate body, and its inclusion in the trunk is secondary. This instructive and very interesting fact is entirely proved by the concordant evidence of comparative anatomy and ontogeny. The lungs are outgrowths of the head-gut; the heart develops from its inner wall. The pleural sacs that enclose the lungs are dorsal parts of the head-cœlom, originating from the pleuroducts; the pericardium in which the heart afterwards lies is also double originally, being formed from ventral halves of the head-cœlom, which only combine at a later stage. When the lung of the air-breathing Vertebrates issues from the head-cavity and enters the trunk-cavity, it follows the example of the floating bladder of the fishes, which also originates from the pharyngeal wall in the shape of a small pouch-like out-growth, but soon grows so large that, in order to find room, it has to pass far behind into the trunk-cavity. To put it more precisely, the lung of the quadrupeds retains this hereditary growth-process of the fishes; for the hydrostatic floating bladder of the latter is the air-filled organ from which the air-breathing organ of the former has been evolved.
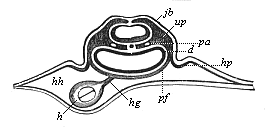
Fig. 382—Transverse section of the cardiac region of the same chick-embryo (behind the preceding). In the cervical cavity (hh) the heart (h) is still connected by a mesocard (hg) with the gut-fibre layer (pf). d gut-gland layer, up provertebral plates, jb rudimentary auditory vesicle in the horny plate, hp first rise of the amniotic fold. (From Remak.)
There is an interesting cenogenetic phenomenon in the formation of the heart of the higher Vertebrates that deserves special notice. In its earliest form the heart is double, as recent observation has shown, in all the Amniotes, and the simple spindle-shaped cardiac tube, which we took as our starting-point, is only formed at a later stage, when the two lateral tubes move backwards, touch each other, and at last combine in the middle line. In man, as in the rabbit, the two embryonic hearts are still far apart at the stage when there are already eight primitive segments (Fig. 134 h). So also the two cœlom-pouches of the head in which they lie are still separated by a broad space. It is not until the permanent body of the embryo develops and detaches from the embryonic vesicle that the separate lateral structures join together, and finally combine in the middle line. As the median partition between the right and left cardiocœl disappears, the two cervical cavities freely communicate (Fig. 381), and form, on the ventral side of the amniote head, a horseshoe-shaped arch, the points of which advance backwards into the pleuro-ducts or pleural cavities, and from there into the two peritoneal sacs of the trunk. But even after the conjunction of the cervical cavities (Fig. 381) the two cardiac tubes remain separate at first; and even after they have united a delicate partition in the middle of the simple endothelial tube (Figs. 379 s, 382 h) indicates the original separation. This cenogenetic “primary cardiac septum” presently disappears, and has no relation to the subsequent permanent partition between the halves of the heart, which, as a heritage from the reptiles, has a great palingenetic importance.
Thorough opponents of the biogenetic law have laid great stress on these and similar cenogenetic phenomena, and endeavoured to urge them as striking disproofs of the law. As in every other instance, careful, discriminating, comparative-morphological examination converts these supposed disproofs of evolution into strong arguments in its favour. In his excellent work, On the structure of the Heart in the Amphibia (1886), Carl Rabl has shown how easily these curious cenogenetic facts can be explained by the secondary adaptation of the embryonic structure to the great extension of the food-yelk.
The embryology of all the other parts of the vascular system also gives us abundant and valuable data for the purposes of phylogeny. But as one needs a thorough knowledge of the intricate structure of the whole vascular system in man and the other Vertebrates in order to follow this with profit, we cannot go into it further here. Moreover, many important features in the ontogeny of the vascular system are still very obscure and controverted. The characters of the embryonic circulation of the Amniotes, which we have previously considered (Chapter XV), are late acquisitions and entirely cenogenetic. (Cf. pp. 170–171; Figs. 198–202.)
If we measure the importance of the systems of organs in the animal frame according to the richness and variety of their phenomena and the physiological interest that this implies, we must regard as one of the principal and most interesting systems the one which we are now going to examine—the system of the reproductive organs. Just as nutrition is the first and most urgent condition for the self-maintenance of the individual organism, so reproduction alone secures the maintenance of the species—or, rather, the maintenance of the long series of generations which the totality of the organic stem represents in their genealogical connection. No individual organism has the prerogative of immortality. To each is allotted only a brief span of personal development, an evanescent moment in the million-year course of the history of life.
Hence, reproduction and the correlative phenomenon, heredity, have long been regarded, together with nutrition, as the most important and fundamental function of living things, and it has been attempted to distinguish them from “lifeless bodies” on this very score. As a matter of fact, this division is not so profound and thorough as it seems to be, and is generally supposed to be. If we examine carefully the nature of the reproductive process, we soon see that it can be reduced to a general property that is found in inorganic as well as organic bodies—growth. Reproduction is a nutrition and growth of the organism beyond the individual limit, which raises a part of it into the whole. This is most clearly seen when we study it in the simplest and lowest organisms, especially the Monera (Figs. 226–228) and the unicellular Amœbæ (Fig. 17). There the simple individual is a single plastid. As soon as it has reached a certain limit of size by continuous feeding and normal growth, it cannot pass it, but divides, by simple cleavage, into two equal halves. Each of these halves then continues its independent life, and grows on until it in turn reaches the limit of growth, and divides. In each of these acts of self-cleavage two new centres of attraction are formed for the particles of bodies, the foundations of the two new-formed individuals. There is no such thing as immortality even in these unicellulars. The individual as such is annihilated in the act of cleavage (cf. p. 48).
In many other Protozoa reproduction takes place not by cleavage, but by budding (gemmation). In this case the growth that determines reproduction is not total (as in segmentation), but partial. Hence in gemmation also we may oppose the local growth-product, that becomes a new individual in the bud, as a child-organism to the parent-organism from which it is formed. The latter is older and larger than the former. In cleavage the two products are equal in age and morphological value. Next to gemmation we have, as other forms of asexual reproduction, the forming of embryonic buds and the forming of embryonic cells. But the latter leads us at once to sexual generation, the distinctive feature of which is the separation of the sexes. I have dealt fully with these various types of reproduction in my History of Creation (chap. viii) and my Wonders of Life (chap. xi).
The earliest ancestors of man and the higher animals had no faculty of sexual reproduction, but multiplied solely by asexual means—cleavage, gemmation, or the formation of embryonic buds or cells, as many Protozoa still do. The differentiation of the sexes came at a later stage. We see this most plainly in the Protists, in which the union of two individuals precedes the continuous cleavage of the unicellular organism (transitory conjugation and permanent copulation of the Infusoria). We may say that in this case the growth (the condition of reproduction) is attained by the coalescence of two full-grown cells into a single, disproportionately large individual. At the same time, the mixture of the two plastids causes a rejuvenation of the plasm. At first the copulating cells are quite homogeneous; but natural selection soon brings about a certain contrast between them—larger female cells (macrospores) and smaller male cells (microspores). It must be a great advantage in the struggle for life for the new individual to have inherited different qualities from the two cellular parents. The further advance of this contrast between the generating cells led to sexual differentiation. One cell became the female ovum (macrogonidion), and the other the male sperm-cell (microgonidion).
The simplest forms of sexual reproduction among the living Metazoa are seen in the Gastræads p. 233, the lower sponges, the common fresh-water polyp (Hydra), and other Cœlenteria of the lowest rank. Prophysema (Fig. 234), Olynthus (Fig. 238), Hydra, etc., have very simple tubular bodies, the thin wall of which consists (as in the original gastrula) only of the two primary germinal layers. As soon as the body reaches sexual maturity, a number of the cells in its wall become female ova, and others male sperm-cells: the former become very large, as they accumulate a considerable quantity of yelk-granules in their protoplasm (Fig. 235 e); the latter are very small on account of their repeated cleavage, and change into mobile cone-shaped spermatozoa (Fig. 20). Both kinds of cells detach from their source of origin, the primary germinal layers, fall either into the surrounding water or into the cavity of the gut, and unite there by fusing together. This is the momentous process of fecundation, which we have examined in Chapter VII (cf. Figs. 23–29).
From these simplest forms of sexual propagation, as we can observe them to-day in the lowest Zoophytes, the Gastræads, Sponges, and Polyps, we gather most important data. In the first place, we learn that, properly speaking, nothing is required for sexual reproduction except the fusion or coalescence of two different cells—a female ovum and male sperm-cell. All other features, and all the very complex phenomena that accompany the sexual act in the higher animals, are of a subordinate and secondary character, and are later additions to this simple, primary process of copulation and fecundation. But if we bear in mind how extremely important a part this relation of the two sexes plays in the whole of organic nature, in the life of plants, of animals, and of man; how the mutual attraction of the sexes, love, is the mainspring of the most remarkable processes—in fact, one of the chief mechanical causes of the highest development of life—we cannot too greatly emphasise this tracing of love to its source, the attractive force of two erotic cells.
Throughout the whole of living nature the greatest effects proceed from this very small cause. Consider the part that the flowers, the sexual organs of the flowering plants, play in nature; or the exuberance of wonderful phenomena that sexual selection produces in animal life; or the momentous influence of love in the life of man. In every case the fusion of two cells is the sole original motive power; in every case this invisible process profoundly affects the development of the most varied structures. We may say, indeed, that no other organic process can be compared to it for a moment in comprehensiveness and intensity of action. Are not the Semitic myth of Adam and Eve, the old Greek legend of Paris and Helena, and so many other famous traditions, only the poetic expression of the vast influence that love and sexual selection have exercised over the course of history ever since the differentiation of the sexes? All the other passions that agitate the heart of man are far outstripped in their joint influence by this sense-inflaming and mind-benumbing Eros. On the one hand, we look to love with gratitude as the source of the greatest artistic achievements—the noblest creations of poetry, plastic art, and music; we see in it the chief factor in the moral advance of humanity, the foundation of family life, and therefore of social advance. On the other hand, we dread it as the devouring flame that brings destruction on so many, and has caused more misery, vice, and crime than all the other evils of human life put together. So wonderful is love and so momentous its influence on the life of the soul, or on the different functions of the medullary tube, that here more than anywhere else the “supernatural” result seems to mock any attempt at natural explanation. Yet comparative evolution leads us clearly and indubitably to the first source of love—the affinity of two different erotic cells, the sperm-cell and ovum.[34]
[34] The sensual perception (probably related to smell) of the two copulating sex-cells, which causes their mutual attraction, is a little understood, but very interesting, chemical function of the cell-soul (cf. p. 58 and The Riddle of the Universe, chap. ix.)
The lowest Metazoa throw light on this very simple origin of the intricate phenomena of reproduction, and they also teach us that the earliest sexual form was hermaphrodism, and that the separation of the sexes (by division of labour) is a secondary and later phenomenon. Hermaphrodism predominates in the most varied groups of the lower animals; each sexually-mature individual, each person, contains female and male sexual cells, and is therefore able to fertilise itself and reproduce. Thus we find ova and sperm-cells in the same individual, not only in the lowest Zoophytes (Gastræads, Sponges, and many Polyps), but also in many worms (leeches and earthworms), many of the snails (the common garden and vineyard snails), all the Tunicates, and many other invertebrate animals. All man’s earlier invertebrate ancestors, from the Gastræads up to the Prochordonia, were hermaphrodites; possibly even the earliest Acrania. We have an instructive proof of this in the remarkable circumstance that many genera of fishes are still hermaphrodites, and that it is occasionally found in the higher Vertebrates of all classes (as atavism). We may conclude from this that gonochorism (separation of the sexes) was a later stage in our development. At first, male and female individuals differ only in the possession of one or other kind of gonads; in other respects they were identical, as we still find in the Amphioxus and the Cyclostomes. Afterwards, accessory organs (ducts, etc.) are associated with the primary sexual glands; and much later again sexual selection has given rise to the secondary sexual characters—those differences between the sexes which do not affect the sexual organs themselves, but other parts of the body (such as the man’s beard or the woman’s breast).
The third important fact that we learn from the lower Zoophytes relates to the earliest origin of the two kinds of sexual cells. As in the Gastræads (the lowest sponges and hydroids), in which we find the first beginnings of sexual differentiation, the whole body consists merely of the two primary germinal layers, it follows that the sexual cells also must have proceeded from the cells of these primary layers, either the inner or outer, or from both. This simple fact is extremely important, because the first trace of the ova as well as the spermatozoa is found in the middle germinal layer or mesoderm in the higher animals, especially the Vertebrates. This arrangement is a later development from the preceding (in connection with the secondary formation of the mesoderm).
If we trace the phylogeny of the sexual organs in our earliest Metazoa ancestors, as the comparative anatomy and ontogeny of the lowest Cœlenteria (Cnidaria, Platodaria) exhibit it to us, we find that the first step in advance is the localisation or concentration of the two kinds of sexual cells scattered in the epithelium into definite groups. In the Sponges and lowest Hydropolyps isolated cells are detached from the cell-strata of the two primary germinal layers, and become free sexual cells; but in the Cnidaria and Platodes we find these associated in groups which we call sexual glands (gonads). We can now for the first time speak of sexual organs in the morphological sense. The female germinative glands, which in this simplest form are merely groups of homogeneous cells, are the ovaries (Fig. 241 c). The male germinative glands, which also in their first form consist of a cluster of sperm-cells, are the testicles (Fig. 241 h). In the medusæ, which descend, both ontogenetically and phylogenetically, from the more simply organised Polyps, we find these simple sexual glands sometimes as gastric pouches, sometimes as outgrowths of the radial canals that proceed from the stomach. Particularly interesting in connection with the question of the first origin of the gonads are the lowest forms of the Platodes, the Cryptocœla that have of late been separated as a special class (Platodaria) from the Turbellaria proper (Fig. 239). In these very primitive Platodes the two pairs of sexual glands are merely two pairs of rows of differentiated cells in the entodermic wall of the primitive gut—two median ovaries (o) within, and two lateral spermaries (s) without. The mature sexual cells are ejected by the posterior outlets; the female (f) lies in front of the male (m).
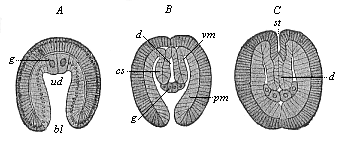
Fig. 383—Embryos of Sagitta, in three earlier stages of development. (From Hertwig.) A gastrula, B cœlomula with open primitive mouth, C the same primitive mouth closed, ua primitive gut, bl primitive mouth, g progonidia (hermaphroditic primitive sexual cells), cs cœlom-pouches, pm parietal layer, vm visceral layer of same, d permanent gut (enteron), st mouth-pit (stomodæum).
In the great majority of the Bilateria or Cœlomaria it is the mesoderm from which the gonads develop. Probably the first traces of them are the two large cells that appear at the edge of the primitive mouth (right and left), as a rule during gastrulation or immediately afterwards—the important promesoblasts, or “polar cells of the mesoderm,” or “primitive cells of the middle germinal layer” (p. 194). In the real Enterocœla, in which the mesoderm appears from the first in the shape of a couple of cœlom-pouches, these are very probably the original gonads (p. 194). This is seen very clearly in the arrow-worm (Sagitta). In the gastrula of Sagitta (Fig. 383 A) we find at an early stage a couple of entodermic cells of an unusual size (g) at the base of the primitive gut (ud). These primitive sexual cells (progonidia) are symmetrically placed to the right and left of the middle plane, like the two promesoblasts of the bilateral gastrula of the Amphioxus (Fig. 38 p). A little outwards from them the two cœlom pouches (B, cs) are developed out of the primitive gut, and each progonidion divides into a male and a female sexual cell (B, g). The two male cells (at first rather the larger) lie close together within, and are the parent-cells of the testicles (prospermaria). The two female cells lie outwards from these, and are the parent-cells of the ovary (protovaria). Afterwards, when the cœlom-pouches have detached from the permanent gut (C, d) and the primitive mouth (A, bl) is closed, the female cells advance towards the mouth (C, st), and the male towards the rear. The foremost pair of ovaries are then separated by a transverse partition from the hind pair. Thus the first structures of the sexual glands of the Sagitta are a couple of hermaphroditic entodermic cells; each of these divides into a male and a female cell; and these four cells are the parent-cells of the four sexual glands. Probably the two promesoblasts of the Amphioxus-gastrula (Fig. 38) are also hermaphroditic primitive sexual cells in the same sense, inherited by this earliest vertebrate from its ancient bilateral gastræad ancestors.
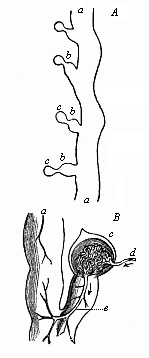
Fig. 384—A, Part of the kidneys of Bdellostoma. a prorenal duct (nephroductus), b segmental or primitive urinary canals (pronephridia), c renal or Malpighian capsules. B Portion of same, highly magnified. c renal capsules with the glomerulus, d afferent artery, e efferent artery. From Johannes Müller (Myxinoides).
The sexually-mature Amphioxus is not hermaphroditic, as its nearest invertebrate relatives, the Tunicates, are, and as the long-extinct pre-Silurian Primitive Vertebrate (Prospondylus, Figs. 98–102) probably was. The actual lancelet has gonochoristic structures of a very interesting kind. As we saw in the anatomy of the Amphioxus, we find the ovaries of the female and the spermaries of the male in the shape of twenty to thirty pairs of elliptical or roundish four-cornered sacs, which lie on either side of the gut on the parietal surface of the respiratory pore (Fig. 219 g). According to the important discovery of Rückert (1888), the sexual glands of the earliest fishes, the Selachii, are similarly arranged. They only unite afterwards to form a pair of simple gonads. These have been transmitted by heredity to all the rest of the Craniotes. In every case they lie originally on each side of the mesentery, underneath the chorda, at the bottom of the body-cavity. The first traces of them are found in the cœlom-epithelium, at the spot where the skin-fibre layer and gut-fibre layer meet in the middle of the mesenteric plate (Fig. 93 mp). At this point we observe at an early stage in all craniote embryos a small string-like cluster of cells, which we may call, with Waldeyer, the “germ epithelium,” or (in harmony with the other plate-shaped rudimentary organs) the sexual plate (Fig. 173 g). This germinal or sexual plate is found in the fifth week in the human embryo, in the shape of a couple of long whitish streaks, on the inner side of the primitive kidneys (Fig. 183 t). The cells of this sexual plate are distinguished by their cylindrical form and chemical composition from the rest of the cœlom-cells; they have a different purport from the flat cells which line the rest of the body-cavity. As the germ epithelium of the sexual plate becomes thicker, and supporting tissue grows into it from the mesoderm, it becomes a rudimentary sexual gland. This ventral gonad then develops into the ovary in the female Craniotes, and the testicles in the male.
In the formation of the gonidia or erotic sexual cells and their conjunction at fecundation we have the sole essential features of sexual reproduction; but in the great majority of animals we find other organs taking part in it. The chief of these secondary sexual organs are the gonoducts, which serve to convey the mature sexual cells out of the body, and the copulative organs, which bring the fecundating male sperm into touch with the ovum-bearing female. The latter organs are, as a rule, only found in the higher animals, and are much less widely distributed than the gonoducts. But these also are secondary formations, and are wanting in many animals of the lower groups.
In the lower animals the mature sexual cells are generally ejected directly from the body. Sometimes they pass out immediately through the skin (Hydra and many hydroids); sometimes they fall into the gastric cavity, and are evacuated by the mouth (gastræads, sponges, many medusæ, and corals); sometimes they fall into the body-cavity, and are ejected by a special pore (porus genitalis) in the ventral wall. The latter procedure is found in many of the worms, and also in the lowest Vertebrates. Amphioxus has the peculiar feature that the mature sexual products fall first into the mantle-cavity; from there they are either evacuated by the respiratory pore, or else they pass through the gill-clefts into the branchial gut, and so out by the mouth (p. 185). In the Cyclostomes they fall into the body-cavity, and are ejected by a genital pore in its wall; so also in some of the fishes. From these we gather the features of our earlier ancestors in this respect. On the other hand, in all the higher and most of the lower Vertebrates (and most of the higher Invertebrates) we find in both sexes special tubular passages of the sexual gland, which are called “gonoducts.” In the female they conduct the ova from the ovary, and so are called “oviducts,” or “Fallopian tubes.” In the male they convey the spermatozoa from the testicles, and are called “spermaducts,” or vasa deferentia.
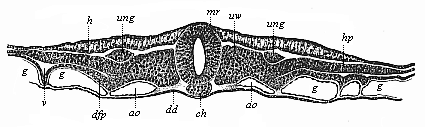
Fig. 385—Transverse section of the embryonic shield of a chick, forty-two hours old. (From Kölliker.) mr medullary tube, ch chorda, h horny plate (skin-sense layer), ung nephroduct, vw episomites (dorsal primitive segments), hp skin-fibre layer (parietal layer of the hyposomites), dfp gut-fibre layer (visceral layer of hyposomites), ao aorta, g vessels. (Cf. transverse section of duck-embryo, Fig. 152.)
The original and genetic relation of these two kinds of ducts is just the same in man as in the rest of the higher Vertebrates, and quite different from what we find in most of the Invertebrates. In the latter, as a rule, the gonoducts develop directly from the embryonic glands or from the outer skin; but in the Vertebrates an independent organic system is employed to convey the sexual products, and this had originally a totally different function—namely, the system of urinary organs. These organs have primarily the sole duty of removing unusable matter from the body in a fluid form. Their liquid excretory product, the urine, is either evacuated directly through the skin or through the last section of the gut. It is only at a later stage that the tubular urinary passages also convey the sexual products from the body. In this way they become “urogenital ducts.” This remarkable secondary conjunction of the urinary and sexual organs into a common urogenital system is very characteristic of the Gnathostomes, the six higher classes of Vertebrates. It is wanting in the lower classes. In order to appreciate it fully, we must give a comparative glance at the structure of the urinary organs.
The renal or urinary system is one of the oldest and most important systems of organs in the differentiated animal body, as I have pointed out on several previous occasions (cf. Chapter XVII). We find it not only in the higher stems, but also very generally distributed in the earlier group of the Vermalia. Here we meet it in the lowest worms, the Rotatoria (Gastrotricha, Fig. 242), and in the instructive stem of the Platodes. It consists of a pair of simple or branching canals, which are lined with one layer of cells, absorb unusable juices from the tissue, and eject them by an outlet in the outer skin (Fig. 240 nm). Not only the free-living Turbellaria, but also the parasitic Suctoria, and even the still more degenerate tapeworms, which have lost their alimentary canal in consequence of their parasitic life, are equipped with these renal canals or nephridia. In the first embryonic structure they are merely a pair of simple cutaneous glands, or depressions in the ectoderm. They are generally described as excretory organs in the worms, but formerly often as “water vessels.” They may be conceived as largely-developed tubular cutaneous glands, formed by invagination of the cutaneous layer. According to another view, they owe their origin to a later rupture of the body-cavity outwards. In most of the Vermalia each nephridium has an inner opening (with cilia) into the body-cavity and an outer one on the epidermis.
Fig. 386—Rudimentary primitive kidneys of a dog-embryo. The hind end of the embryonic body is seen from the ventral side and covered with the visceral layer of the yelk-sac, which is torn away and folded down in front in order to show the nephroducts with the primitive urinary canals (a). b primitive vertebræ, c spinal cord, d entrance into the pelvic-gut cavity. (From Bischoff.)
Fig. 387—Primitive kidneys of a human embryo. u the urinary canals of the primitive kidneys, w Wolffian duct, w′ uppermost end of the same (Morgagni’s hydatid), m Mullerian duct. m′ uppermost end of same (Fallopian hydatid), g gonad (sexual gland). (From Kobelt.)
In these lowest, unsegmented worms, and in the unsegmented Molluscs, there is only one pair of renal canals. They are more numerous in the higher Articulates. In the Annelids, the body of which is composed of a large number of joints, there is a pair of these pronephridia in each segment (hence they are called segmental canals or organs). Even here they are still simple tubes; on account of their coiled or looped form they are often called “looped canals.” In most of the Annelids, and many of the Vermalia, we can distinguish three sections in the nephridium—an outer muscular duct, a glandular middle part, and an inner part that opens by a ciliated funnel into the body-cavity. This opening is furnished with whirling cilia, and can, therefore, take up the juices to be excreted directly from the body-cavity and convey them from the body. But in these worms the sexual cells, which develop in very primitive form on the inner surface of the body-cavity, also fall into it when mature, and are sucked up by the funnel-shaped inner ciliated openings of the renal canals, and ejected with the urine. Thus the urine-forming looped canals, or pronephridia, serve as oviducts in the female Annelids and as spermaducts in the male.
The renal system of the Vertebrates is similar to, yet materially different from, these segmental canals of the Annelids. The peculiar development of it and its relations to the sexual organs are among the most difficult problems in the morphology of our stem. If we examine briefly the vertebrate renal system from the phylogenetic point of view, as confirmed by recent discoveries, we may distinguish three forms of it: (1) Fore-kidneys or head-kidneys (pronephros); (2) primitive or middle kidneys (mesonephros); (3) permanent kidneys (metanephros). These three systems of kidneys are not fundamentally and completely distinct, as earlier students (such as Semper) wrongly supposed; they represent three different generations of one and the same excretory apparatus; they correspond to three phylogenetic stages, and succeed each other in the stem-history of the Vertebrates in such wise that each younger and more advanced generation develops farther behind in the body, and replaces the older and less advanced generation that preceded it in time and space. The fore kidneys, first accurately described by Wilhelm Müller in 1875 in the Cyclostomes and Ichthyoda, form the sole excretory organ of the Acrania (Amphioxus); they continue in the Cyclostomes and some of the fishes, but are found only in slight traces and for a time in the embryos of the six other classes of Vertebrates. The primitive kidneys are first found in the Cyclostomes, behind the fore kidneys; they have been transmitted from the Selachii to all the Gnathostomes. In the Anamnia they act permanently as urinary glands; in the Amniotes their anterior part (“germinal kidneys”) changes into organs of the sexual apparatus, while the third generation develops from the end of their posterior part (“urinal kidneys”)—the characteristic after or permanent kidneys of the three higher classes of Vertebrates. The order in which the three renal systems succeed each other in the embryo of man and the higher Vertebrates corresponds to their phylogenetic succession in the history of our stem, and, consequently, in the natural classification of the Vertebrates.
Fig. 388—Pig-embryo, three-fifths of an inch long, seen from the ventral side. a fore leg, z hind leg, b ventral wall, r sexual prominence, w nephroduct, n primitive kidneys, n1 their inner part. (From Oscar Schultze.)
Fig. 389—Human embryo of the fifth week, two-fifths of an inch long, seen from the ventral side (the anterior ventral wall, b, is removed, the body-cavity, c, opened). d gut (cut off), f frontal process, g cerebrum, m middle brain, e after brain, h heart, k first gill-cleft, l pulmonary sac, n primitive kidneys, r sexual region, p phallus (sexual prominences), s tail. (From Kollmann.)
As in the morphology of any other system of organs, so in the case of the urinary and sexual organs the Amphioxus is the real typical primitive Vertebrate; it affords the key to the mysteries of the structure of man and the higher Vertebrates. The kidneys of the Amphioxus—first discovered by Boveri in 1890—are typical “fore kidneys,” composed of a double row of short segmental canals (Fig. 217 x). The inner aperture of these pronephridia opens into the mesodermic body-cavity (the middle part of the cœloma, B); the external aperture into the ectodermic mantle or peribranchial cavity (C). Their position, their structure, and their relation to the branchial vessel make it clear that these segmental pronephridia correspond to the rudimentary fore kidneys of the Craniotes. The mantle-cavity into which they open seems to correspond to the prorenal duct of the latter.
Figs. 390, 391, 392—Primitive kidneys and rudimentary sexual organs. Figs. 390 and 391 of Amphibia (frog-larvæ); Fig. 390 earlier, 391 later stage. Fig. 392 of a mammal (ox-embryo). u primitive kidney, k sexual gland (rudiment of testicle and ovary). The primary nephroduct (ug in Fig. 390) divides (in Figs. 391 and 392) into the two secondary nephroducts—the Mullerian (m) and Wolffian (ug′) ducts, joined together behind in the genital cord (g). l ligament of the primitive kidneys. (From Gegenbaur.)
Figs. 393, 394—Urinary and sexual organs of an Amphibian (water salamander or Triton). Fig. 393 of a female, 394 of a male. r primitive kidney, ov ovary, od oviduct and c Rathke’s duct, both developed from the Müllerian duct, u primitive ureter (also acting as spermaduct [ve] in the male, opening below into the Wolffian duct [u apostrophe]), ms mesovarium. (From Gegenbaur.)
The next higher Vertebrates, the Cyclostomes, yield some very interesting data. Both orders of this class, the hags and lampreys, have still the fore kidneys inherited from the Acrania—the former permanently, the latter in their earlier stages. Behind these the primitive kidneys soon develop, and in a very characteristic form. The remarkable structure of the mesonephros of the Cyclostomes, discovered by Johannes Müller, explains the intricate formation of the kidneys in the higher Vertebrates. We find in the hag-fishes (Bdellostoma) a long tube, the prorenal duct (nephroductus, Fig. 384 a). This opens with its anterior end into the cœloma by a ciliated aperture, and externally with its posterior end by an outlet in the skin. Inside it open a large number of small transverse canals (“segmental or primitive urinary canals,” b). Each of these terminates blindly in a vesicular capsule (c), and this encloses a coil of blood-vessel (glomerulus, an arterial network, Fig. 384 B, c). Afferent branches of arteries conduct arterial blood into the coiled branches of the glomerulus (d), and efferent arterial branches conduct it away from the net (c). The primitive renal canals (mesonephridia) are distinguished by this net-formation from their predecessors.
In the Selachii also we find a longitudinal row of segmental canals on each side, which open outwards into the primitive renal ducts (nephrotomes, p. 149. The segmental canals (a pair in each segment of the middle part of the body) open internally by a ciliated funnel into the body-cavity. From the posterior group of these organs a compact primitive kidney is formed, the anterior group taking part in the construction of the sexual organs.
In the same simple form that remains throughout life in the Myxinoides and partly in the Selachii we find the primitive kidney first developing in the embryo of man and the higher Craniotes (Figs. 386, 387). Of the two parts that compose the comb-shaped primitive kidney the longitudinal channel, or nephroduct, is always the first to appear; afterwards the transverse “canals,” the excreting nephridia, are formed in the mesoderm; and after this again the Malpighian capsules with their arterial coils are associated with these as cœlous outgrowths. The primitive renal duct, which appears first, is found in all craniote embryos at the early stage in which the differentiation of the medullary tube takes place in the ectoderm, the severance of the chorda from the visceral layer in the entoderm, and the first trace of the cœlom-pouches arises between the limiting layers (Fig. 385). The nephroduct (ung) is seen on each side, directly under the horny plate, in the shape of a long, thin, thread-like string of cells. It presently hollows out and becomes a canal, running straight from front to back, and clearly showing in the transverse section of the embryo its original position in the space between horny plate (h), primitive segments (uw), and lateral plates (hpl). As the originally very short urinary canals lengthen and multiply, each of the two primitive kidneys assumes the form of a half-feathered leaf (Fig. 387). The lines of the leaf are represented by the urinary canals (u), and the rib by the outlying nephroduct (w). At the inner edge of the primitive kidneys the rudiment of the ventral sexual gland (g) can now be seen as a body of some size. The hindermost end of the nephroduct opens right behind into the last section of the rectum, thus making a cloaca of it. However, this opening of the nephroducts into the intestine must be regarded as a secondary formation. Originally they open, as the Cyclostomes clearly show, quite independently of the gut, in the external skin of the abdomen.
Fig. 395—Primitive kidneys and germinal glands of a human embryo, three inches in length (beginning of the sixth week), magnified. k germinal gland, u primitive kidney, z diaphragmatic ligament of same, w Wolffian duct (opened on the right), g directing ligament (gubernaculum), a allantoic duct. (From Kollmann.)
In the Myxinoides the primitive kidneys retain this simple comb-shaped structure, and a part of it is preserved in the Selachii; but in all the other Craniotes it is only found for a short time in the embryo, as an ontogenetic reproduction of the earlier phylogenetic structure. In these the primitive kidney soon assumes the form (by the rapid growth, lengthening, increase, and serpentining of the urinary canals) of a large compact gland, of a long, oval or spindle-shaped character, which passes through the greater part of the embryonic body-cavity (Figs. 183 m, 184 m, 388 n). It lies near the middle line, directly under the primitive vertebral column, and reaches from the cardiac region to the cloaca. The right and left kidneys are parallel to each other, quite close together, and only separated by the mesentery—the thin narrow layer that attaches the middle gut to the under surface of the vertebral column. The passage of each primitive kidney, the nephroduct, runs towards the back on the lower and outer side of the gland, and opens in the cloaca, close to the starting-point of the allantois; it afterwards opens into the allantois itself.
The primitive or primordial kidneys of the amniote embryo were formerly called the “Wolffian bodies,” and sometimes “Oken’s bodies.” They act for a time as kidneys, absorbing unusable juices from the embryonic body and conducting them to the cloaca—afterwards to the allantois. There the primitive urine accumulates, and thus the allantois acts as bladder or urinary sac in the embryos of man and the other Amniotes. It has, however, no genetic connection with the primitive kidneys, but is a pouch-like growth from the anterior wall of the rectum (Fig. 147 u). Thus it is a product of the visceral layer, whereas the primitive kidneys are a product of the middle layer. Phylogenetically we must suppose that the allantois originated as a pouch-like growth from the cloaca-wall in consequence of the expansion caused by the urine accumulated in it and excreted by the kidneys. It is originally a blind sac of the rectum. The real bladder of the vertebrate certainly made its first appearance among the Dipneusts (in Lepidosiren), and has been transmitted from them to the Amphibia, and from these to the Amniotes. In the embryo of the latter it protrudes far out of the not yet closed ventral wall. It is true that many of the fishes also have a “bladder.” But this is merely a local enlargement of the lower section of the nephroducts, and so totally different in origin and composition from the real bladder. The two structures can be compared from the physiological point of view, and so are analogous, as they have the same function; but not from the morphological point of view, and are therefore not homologous. The false bladder of the fishes is a mesodermic product of the nephroducts; the true bladder of the Dipneusts, Amphibia, and Amniotes is an entodermic blind sac of the rectum.
Figs. 396–398—Urinary and sexual organs of ox-embryos. Fig. 396, female embryo one and a half inches long; Fig. 397, male embryo, one and a half inches long. Fig. 398 female embryo two and a half inches long. w primitive kidney, wg Wolffian duct, m Müllerian duct, m′ upper end of same (opened at t), i lower and thicker part of same (rudiment of uterus), g genital cord, h testicle, (h′, lower and h″, upper testicular ligament), o ovary, o′ lower ovarian ligament, i inguinal ligament of primitive kidney, d diaphragmatic ligament of primitive kidney, nn accessory kidneys, n permanent kidneys, under them the S-shaped ureters, between these the rectum, v bladder, a umbilical artery. (From Kölliker.)
In all the Anamnia (the lower amnionless Craniotes, Cyclostomes, Fishes, Dipneusts, and Amphibia) the urinary organs remain at a lower stage of development to this extent, that the primitive kidneys (protonephri) act permanently as urinary glands. This is only so as a passing phase of the early embryonic life in the three higher classes of Vertebrates, the Amniotes. In these the permanent or after or secondary (really tertiary) kidneys (renes or metanephri) that are distinctive of these three classes soon make their appearance. They represent the third and last generation of the vertebrate kidneys. The permanent kidneys do not arise (as was long supposed) as independent glands from the alimentary tube, but from the last section of the primitive kidneys and the nephroduct. Here a simple tube, the secondary renal duct, develops, near the point of its entry into the cloaca; and this tube grows considerably forward. With its blind upper or anterior end is connected a glandular renal growth, that owes its origin to a differentiation of the last part of the primitive kidneys. This rudiment of the permanent kidneys consists of coiled urinary canals with Malpighian capsules and vascular coils (without ciliated funnels), of the same structure as the segmental mesonephridia of the primitive kidneys. The further growth of these metanephridia gives rise to the compact permanent kidneys, which have the familiar bean-shape in man and most of the higher mammals, but consist of a number of separate folds in the lower mammals, birds, and reptiles. As the permanent kidneys grow rapidly and advance forward, their passage, the ureter, detaches altogether from its birth-place, the posterior end of the nephroduct; it passes to the posterior surface of the allantois. At first in the oldest Amniotes this ureter opens into the cloaca together with the last section of the nephroduct, but afterwards separately from this, and finally into the permanent bladder apart from the rectum altogether. The bladder originates from the hindmost and lowest part of the allantoic pedicle (urachus), which enlarges in spindle shape before the entry into the cloaca. The anterior or upper part of the pedicle, which runs to the navel in the ventral wall of the embryo, atrophies subsequently, and only a useless string-like relic of it is left as a rudimentary organ; that is the single vesico-umbilical ligament. To the right and left of it in the adult male are a couple of other rudimentary organs, the lateral vesico-umbilical ligaments. These are the degenerate string-like relics of the earlier umbilical arteries.
Though in man and all the other Amniotes the primitive kidneys are thus early replaced by the permanent kidneys, and these alone then act as urinary organs, all the parts of the former are by no means lost. The nephroducts become very important physiologically by being converted into the passages of the sexual glands. In all the Gnathostomes—or all the Vertebrates from the fishes up to man—a second similar canal develops beside the nephroduct at an early stage of embryonic evolution. The latter is usually called the Müllerian duct, after its discoverer, Johannes Müller, while the former is called the Wolffian duct. The origin of the Müllerian duct is still obscure; comparative anatomy and ontogeny seem to indicate that it originates by differentiation from the Wolffian duct. Perhaps it would be best to say: “The original primary nephroduct divides by differentiation (or longitudinal cleavage) into two secondary nephroducts, the Wolffian and the Müllerian ducts.” The latter (Fig. 387 m) lies just on the inner side of the former (Fig. 387 w). Both open behind into the cloaca.
Fig. 399—Female sexual organs of a Monotreme (Ornithorhynchus, Fig. 269). o ovaries, t oviducts, u womb, sug urogenital sinus; at u′ is the outlet of the two wombs, and between them the bladder (vu). cl cloaca. (From Gegenbaur.)
However uncertain the origin of the nephroduct and its two products, the Müllerian and the Wolffian ducts, may be, its later development is clear enough. In all the Gnathostomes the Wolffian duct is converted into the spermaduct, and the Müllerian duct into the oviduct. Only one of them is retained in each sex; the other either disappears altogether, or only leaves relics in the shape of rudimentary organs. In the male sex, in which the two Wolffian ducts become the spermaducts, we often find traces of the Müllerian ducts, which I have called “Rathke’s canals” (Fig. 394 c). In the female sex, in which the two Müllerian ducts form the oviducts, there are relics of the Wolffian ducts, which are called “the ducts of Gaertner.”
We obtain the most interesting information with regard to this remarkable evolution of the nephroducts and their association with the sexual glands from the Amphibia (Figs. 390–395). The first structure of the nephroduct and its differentiation into Müllerian and Wolffian ducts are just the same in both sexes in the Amphibia, as in the mammal embryos (Figs. 392, 396). In the female Amphibia the Müllerian duct develops on either side into a large oviduct (Fig. 393 od), while the Wolffian duct acts permanently as ureter (u). In the male Amphibia the Müllerian duct only remains as a rudimentary organ without any functional significance, as Rathke’s canal (Fig. 394 c); the Wolffian duct serves also as ureter, but at the same time as spermaduct, the sperm-canals (ve) that proceed from the testicles (t) entering the fore part of the primitive kidneys and combining there with the urinary canals.
Figs. 400, 401—Original position of the sexual glands in the ventral cavity of the human embryo (three months old). Fig. 400, male. h testicles, gh conducting ligament of the testicles, wg spermaduct, h bladder, uh inferior vena cava, nn accessory kidneys, n kidneys. Fig. 401, female. r round maternal ligament (underneath it the bladder, over it the ovaries). r′ kidneys, s accessory kidneys, c cæcum, o small reticle, om large reticle (stomach between the two), l spleen. (From Kölliker.)
In the mammals these permanent amphibian features are only seen as brief phases of the earlier period of embryonic development (Fig. 392). Here the primitive kidneys, which act as excretory organs of urine throughout life in the amnion-less Vertebrates, are replaced in the mammals by the permanent kidneys. The real primitive kidneys disappear for the most part at an early stage of development, and only small relics of them remain. In the male mammal the epididymis develops from the uppermost part of the primitive kidney; in the female a useless rudimentary organ, the epovarium, is formed from the same part. The atrophied relic of the former is known as the paradidymis, that of the latter as the parovarium.
Fig. 402—Urogenital system of a human embryo of three inches in length. h testicles, wg spermaducts, gh conducting ligament, p processus vaginalis, b bladder, au umbilical arteries, m mesorchium, d intestine, u ureter, n kidney, nn accessory kidney. (From Kollman.)
The Müllerian ducts undergo very important changes in the female mammal. The oviducts proper are developed only from their upper part; the lower part dilates into a spindle-shaped tube with thick muscular wall, in which the impregnated ovum develops into the embryo. This is the womb (uterus). At first the two wombs (Fig. 399 u) are completely separate, and open into the cloaca on either side of the bladder (vu), as is still the case in the lowest living mammals, the Monotremes. But in the Marsupials a communication is opened between the two Müllerian ducts, and in the Placentals they combine below with the rudimentary Wolffian ducts to form a single “genital cord.” The original independence of the two wombs and the vaginal canals formed from their lower ends are retained in many of the lower Placentals, but in the higher they gradually blend and form a single organ. The conjunction proceeds from below (or behind) upwards (or forwards). In many of the Rodents (such as the rabbit and squirrel) two separate wombs still open into the simple and single vaginal canal; but in others, and in the Carnivora, Cetacea, and Ungulates, the lower halves of the wombs have already fused into a single piece, though the upper halves (or “horns”) are still separate (“two-horned” womb, uteris bicornis). In the bats and lemurs the “horns” are very short, and the lower common part is longer. Finally, in the apes and in man the blending of the two halves is complete, and there is only the one simple, pear-shaped uterine pouch, into which the oviducts open on each side. This simple uterus is a late evolutionary product, and is found only in the ape and man.
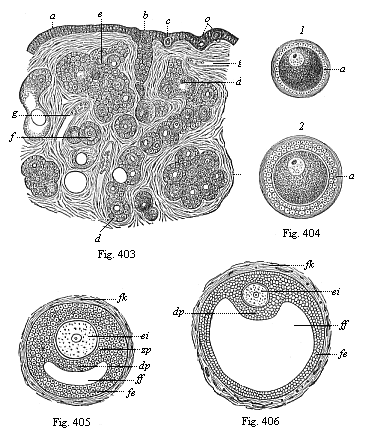
Figs. 403–406—Origin of human ova in the
female ovary. Fig. 403. Vertical section of the ovary of a new-born
female infant, a ovarian epithelium, b rudimentary string of ova,
c young ova in the epithelium, d long string of ova with
follicle-formation (Pflüger’s tube), e group of young follicles,
f isolated young follicle, g blood-vessels in connective tissue
(stroma) of the ovary. In the strings the young ova are distinguished by their
considerable size from the surrounding follicle-cells. (From
Waldeyer.)
Fig. 404—Two young Graafian follicles,
isolated. In 1 the follicle-cells still form a simple, and in 2 a
double, stratum round the young ovum; in 2 they are beginning to form
the ovolemma or the zona pellucida (a).
Figs. 405 and
406—Two older Graafian follicles, in which fluid is beginning to
accumulate inside the eccentrically thickened epithelial mass of the
follicle-cells (Fig. 405 with little, 406 with much, follicle-water). ei
the young ovum, with embryonic vesicle and spot, zp ovolemma or zona
pellucida, dp discus proligerus, formed of an accumulation of
follicle-cells, which surround the ovum, ff follicle-liquid (liquor
folliculi), gathered inside the stratified follicle-epithelium (fe),
fk connective-tissue fibrous capsule of the Graafian follicle (theca
folliculi).
In the male mammals there is the same fusion of the Müllerian and Wolffian ducts at their lower ends. Here again they form a single genital cord (Fig. 397 g), and this opens similarly into the original urogenital sinus, which develops from the lowest section of the bladder (v). But while in the male mammal the Wolffian ducts develop into the permanent spermaducts, there are only rudimentary relics left of the Müllerian ducts. The most notable of these is the “male womb” (uterus masculinus), which originates from the lowest fused part of the ducts, and corresponds to the female uterus. It is a small, flask-shaped vesicle without any physiological significance, which opens into the ureter between the two spermaducts and the prostate folds (vesicula prostatica).
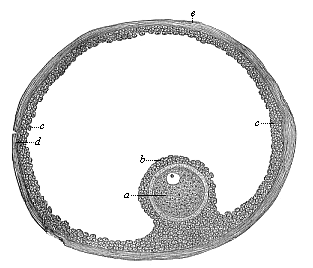
Fig. 407—A ripe human Graafian follicle. a the mature ovum, b the surrounding follicle-cells, c the epithelial cells of the follicle, d the fibrous membrane of the follicle, e its outer surface.
The internal sexual organs of the mammals undergo very distinctive changes of position. At first the germinal glands of both sexes lie deep inside the ventral cavity, at the inner edge of the primitive kidneys (Figs. 386 g, 392 k), attached to the vertebral column by a short mesentery (mesorchium in the male, mesovarium in the female). But this primary arrangement is retained permanently only in the Monotremes (and the lower Vertebrates). In all other mammals (both Marsupials and Placentals) they leave their original cradle and travel more or less far down (or behind), following the direction of a ligament that goes from the primitive kidneys to the inguinal region of the ventral wall. This is the inguinal ligament of the primitive kidneys, known in the male as the Hunterian ligament (Fig. 400 gh), and in the female as the “round maternal ligament” (Fig. 401 r). In woman the ovaries travel more or less towards the small pelvis, or enter into it altogether. In the male the testicles pass out of the ventral cavity, and penetrate by the inguinal canal into a sac-shaped fold of the outer skin. When the right and left folds (“sexual swellings”) join together they form the scrotum. The various mammals bring before us the successive stages of this displacement. In the elephant and the whale the testicles descend very little, and remain underneath the kidneys. In many of the rodents and carnassia they enter the inguinal canal. In most of the higher mammals they pass through this into the scrotum. As a rule, the inguinal canal closes up. When it remains open the testicles may periodically pass into the scrotum, and withdraw into the ventral cavity again in time of rut (as in many of the marsupials, rodents, bats, etc.).
The structure of the external sexual organs, the copulative organs that convey the fecundating sperm from the male to the female organism in the act of copulation, is also peculiar to the mammals. There are no organs of this character in most of the other Vertebrates. In those that live in water (such as the Acrania and Cyclostomes, and most of the fishes) the ova and sperm-cells are simply ejected into the water, where their conjunction and fertilisation are left to chance. But in many of the fishes and amphibia, which are viviparous, there is a direct conveyance of the male sperm into the female body; and this is the case with all the Amniotes (reptiles, birds, and mammals). In these the urinary and sexual organs always open originally into the last section of the rectum, which thus forms a cloaca (p. 249). Among the mammals this arrangement is permanent only in the Monotremes, which take their name from it (Fig. 399 cl). In all the other mammals a frontal partition is developed in the cloaca (in the human embryo about the beginning of the third month), and this divides it into two cavities. The anterior cavity receives the urogenital canal, and is the sole outlet of the urine and the sexual products; the hind or anus-cavity passes the excrements only.
Even before this partition has been formed in the Marsupials and Placentals, we see the first trace of the external sexual organs. First a conical protuberance rises at the anterior border of the cloaca-outlet—the sexual prominence (phallus, Fig. 402 A, e, B, e). At the tip it is swollen in the shape of a club (“acorn” glans). On its under side there is a furrow, the sexual groove (sulcus genitalis, f), and on each side of this a fold of skin, the “sexual pad” (torus genitalis, h l). The sexual protuberance or phallus is the chief organ of the sexual sense (p. 282); the sexual nerves spread on it, and these are the principal organs of the specific sexual sensation. As erectile bodies (corpora cavernosa) are developed in the male phallus by peculiar modifications of the blood-vessels, it becomes capable of erecting periodically on a strong accession of blood, becoming stiff, so as to penetrate into the female vagina and thus effect copulation. In the male the phallus becomes the penis; in the female it becomes the much smaller clitoris; this is only found to be very large in certain apes (Ateles). A prepuce (“foreskin”) is developed in both sexes as a protecting fold on the anterior surface of the phallus.
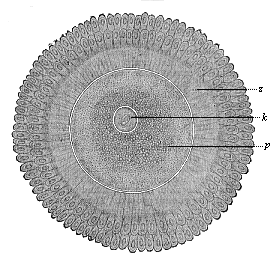
Fig. 408—The human ovum after issuing from the Graafian follicle, surrounded by the clinging cells of the discus proligerus (in two radiating crowns). z ovolemma (zona pellucida, with radial porous canals), p cytosoma (protoplasm of the cell-body, darker within, lighter without), k nucleus of the ovum (embryonic vesicle). (From Nagel.) (Cf. Figs. 1 and 14.)
The external sexual member (phallus) is found at various stages of development within the mammal class, both in regard to size and shape, and the differentiation and structure of its various parts; this applies especially to the terminal part of the phallus, the glans, both the larger glans penis of the male and the smaller glans clitoridis of the female. The part of the cloaca from the upper wall of which it forms belongs to the proctodæum, the ectodermic invagination of the rectum (p. 311); hence its epithelial covering can develop the same horny growths as the corneous layer of the epidermis. Thus the glans, which is quite smooth in man and the higher apes, is covered with spines in many of the lower apes and in the cat, and in many of the rodents with hairs (marmot) or scales (guinea-pig) or solid horny warts (beaver). Many of the Ungulates have a free conical projection on the glans, and in many of the Ruminants this “phallus-tentacle” grows into a long cone, bent hook-wise at the base (as in the goat, antelope, gazelle, etc.). The different forms of the phallus are connected with variations in the structure and distribution of the sensory corpuscles—i.e. the real organs of the sexual sense, which develop in certain papillæ of the corium of the phallus, and have been evolved from ordinary tactile corpuscles of the corium by erotic adaptation (p. 282).
The formation of the corpora cavernosa, which cause the stiffness of the phallus and its capability of penetrating the vagina, by certain special structures of their spongy vascular spaces, also shows a good deal of variety within the vertebrate stem. This stiffness is increased in many orders of mammals (especially the carnassia and rodents) by the ossification of a part of the fibrous body (corpus fibrosum). This penis-bone (os priapi) is very large in the badger and dog, and bent like a hook in the marten; it is also very large in some of the lower apes, and protrudes far out into the glans. It is wanting in most of the anthropoid apes; it seems to have been lost in their case (and in man) by atrophy.
The sexual groove on the under side of the phallus receives in the male the mouth of the urogenital canal, and is changed into a continuation of this, becoming a closed canal by the juncture of its parallel edges, the male urethra. In the female this only takes place in a few cases (some of the lemurs, rodents, and moles); as a rule, the groove remains open, and the borders of this “vestibule of the vagina” develop into the smaller labia (nymphæ). The large labia of the female develop from the sexual pads (tori genitales), the two parallel folds of the skin that are found on each side of the genital groove. They join together in the male, and form the closed scrotum. These striking differences between the two sexes cannot yet be detected in the human embryo of the ninth week. We begin to trace them in the tenth week of development, and they are accentuated in proportion as the difference of the sexes develops.
Sometimes the normal juncture of the two sexual pads in the male fails to take place, and the sexual groove may also remain open (hypospadia). In these cases the external male genitals resemble the female, and they are often wrongly regarded as cases of hermaphrodism. Other malformations of various kinds are not infrequently found in the human external sexual organs, and some of them have a great morphological interest. The reverse of hypospadia, in which the penis is split open below, is seen in epispadia, in which the urethra is open above. In this case the urogenital canal opens above at the dorsal root of the penis; in the former case down below. These and similar obstructions interfere with a man’s generative power, and thus prejudicially affect his whole development. They clearly prove that our history is not guided by a “kind Providence,” but left to the play of blind chance.
We must carefully distinguish the rarer cases of real hermaphrodism from the preceding. This is only found when the essential organs of reproduction, the genital glands of both kinds, are united in one individual. In these cases either an ovary is developed on the right and a testicle on the left (or vice versa); or else there are testicles and ovaries on both sides, some more and others less developed. As hermaphrodism was probably the original arrangement in all the Vertebrates, and the division of the sexes only followed by later differentiation of this, these curious cases offer no theoretical difficulty. But they are rarely found in man and the higher mammals. On the other hand, we constantly find the original hermaphrodism in some of the lower Vertebrates, such as the Myxinoides, many fishes of the perch-type (serranus), and some of the Amphibia (ringed snake, toad). In these cases the male often has a rudimentary ovary at the fore end of the testicle; and the female sometimes has a rudimentary, inactive testicle. In the carp also and some other fishes this is found occasionally. We have already seen how traces of the earlier hemaphrodism can be traced in the passages of the Amphibia.
Man has faithfully preserved the main features of his stem-history in the ontogeny of his urinary and sexual organs. We can follow their development step by step in the human embryo in the same advancing gradation that is presented to us by the comparison of the urogenital organs in the Acrania, Cyclostomes; Fishes, Amphibia, Reptiles, and then (within the mammal series) in the Monotremes, Marsupials, and the various Placentals. All the peculiarities of urogenital structure that distinguish the mammals from the rest of the Vertebrates are found in man; and in all special structural features he resembles the apes, particularly the anthropoid apes. In proof of the fact that the special features of the mammals have been inherited by man, I will, in conclusion, point out the identical way in which the ova are formed in the ovary. In all the mammals the mature ova are contained in special capsules, which are known as the Graafian follicles, after their discoverer, Roger de Graaf (1677). They were formerly supposed to be the ova themselves; but Baer discovered the ova within the follicles (p. 16). Each follicle (Fig. 407) consists of a round fibrous capsule (d), which contains fluid and is lined with several strata of cells (c). The layer is thickened like a knob at one point (b); this ovum-capsule encloses the ovum proper (a). The mammal ovary is originally a very simple oval body (Fig. 387 g), formed only of connective tissue and blood-vessels, covered with a layer of cells, the ovarian epithelium or the female germ epithelium. From this germ epithelium strings of cells grow out into the connective tissue or “stroma” of the ovary (Fig. 403 b). Some of the cells of these strings (or Pflüger’s tubes) grow larger and become ova (primitive ova, c); but the great majority remain small, and form a protective and nutritive stratum of cells round each ovum—the “follicle-epithelium” (e).
The follicle-epithelium of the mammal has at first one stratum (Fig. 404 1), but afterwards several (2). It is true that in all the other Vertebrates the ova are enclosed in a membrane, or “follicle,” that consists of smaller cells. But it is only in the mammals that fluid accumulates between the growing follicle-cells, and distends the follicle into a large round capsule, on the inside wall of which the ovum lies, at one side (Figs. 405, 406). There again, as in the whole of his morphology, man proves indubitably his descent from the mammals.
In the lower Vertebrates the formation of ova in the germ-epithelium of the ovary continues throughout life; but in the higher it is restricted to the earlier stages, or even to the period of embryonic development. In man it seems to cease in the first year; in the second year we find no new-formed ova or chains of ova (Pflüger’s tubes). However, the number of ova in the two ovaries is very large in the young girl; there are calculated to be 72,000 in the sexually-mature maiden. In the production of the ova men resemble most of the anthropoid apes.
Generally speaking, the natural history of the human sexual organs is one of those parts of anthropology that furnish the most convincing proofs of the animal origin of the human race. Any man who is acquainted with the facts and impartially weighs them will conclude from them alone that we have been evolved from the lower Vertebrates. The larger and the detailed structure, the action, and the embryological development of the sexual organs are just the same in man as in the apes. This applies equally to the male and the female, the internal and the external organs. The differences we find in this respect between man and the anthropoid apes are much slighter than the differences between the various species of apes. But all the apes have certainly a common origin, and have been evolved from a long-extinct early-Tertiary stem-form, which we must trace to a branch of the lemurs. If we had this unknown pithecoid stem-form before us, we should certainly put it in the order of the true apes in the primate system; but within this order we cannot, for the anatomic and ontogenetic reasons we have seen, separate man from the group of the anthropoid apes. Here again, therefore, on the ground of the pithecometra-principle, comparative anatomy and ontogeny teach with full confidence the descent of man from the ape.
Now that we have traversed the wonderful region of human embryology and are familiar with the principal parts of it, it will be well to look back on the way we have come, and forward to the further path to truth to which it has led us. We started from the simplest facts of ontogeny, or the development of the individual—from observations that we can repeat and verify by microscopic and anatomic study at any moment. The first and most important of these facts is that every man, like every other animal, begins his existence as a simple cell. This round ovum has the same characteristic form and origin as the ovum of any other mammal. From it is developed in the same manner in all the Placentals, by repeated cleavage, a multicellular blastula. This is converted into a gastrula, and this in turn into a blastocystis (or embryonic vesicle). The two strata of cells that compose its wall are the primary germinal layers, the skin-layer (ectoderm), and gut-layer (entoderm). This two-layered embryonic form is the ontogenetic reproduction of the extremely important phylogenetic stem-form of all the Metazoa, which we have called the Gastræa. As the human embryo passes through the gastrula-form like that of all the other Metazoa, we can trace its phylogenetic origin to the Gastræa.
As we continued to follow the embryonic development of the two-layered structure, we saw that first a third, or middle layer (mesoderm), appears between the two primary layers; when this divides into two, we have the four secondary germinal layers. These have just the same composition and genetic significance in man as in all the other Vertebrates. From the skin-sense layer are developed the epidermis, the central nervous system, and the chief part of the sense-organs. The skin-fibre layer forms the corium and the motor organs—the skeleton and the muscular system. From the gut-fibre layer are developed the vascular system, the muscular wall of the gut, and the sexual glands. Finally, the gut-gland layer only forms the epithelium, or the inner cellular stratum of the mucous membrane of the alimentary canal and glands (lungs, liver, etc.).
The manner in which these different systems of organs arise from the secondary germinal layers is essentially the same from the start in man as in all the other Vertebrates. We saw, in studying the embryonic development of each organ, that the human embryo follows the special lines of differentiation and construction that are only found otherwise in the Vertebrates. Within the limits of this vast stem we have followed, step by step, the development both of the body as a whole and of its various parts. This higher development follows in the human embryo the form that is peculiar to the mammals. Finally, we saw that, even within the limits of this class, the various phylogenetic stages that we distinguish in a natural classification of the mammals correspond to the ontogenetic stages that the human embryo passes through in the course of its evolution. We were thus in a position to determine precisely the position of man in this class, and so to establish his relationship to the different orders of mammals.
The line of argument we followed in this explanation of the ontogenetic facts was simply a consistent application of the biogenetic law. In this we have throughout taken strict account of the distinction between palingenetic and cenogenetic phenomena. Palingenesis (or “synoptic development”) alone enables us to draw conclusions from the observed embryonic form to the stem-form preserved by heredity. Such inference becomes more or less precarious when there has been cenogenesis, or disturbance of development, owing to fresh adaptations. We cannot understand embryonic development unless we appreciate this very important distinction. Here we stand at the very limit that separates the older and the new science or philosophy of nature. The whole of the results of recent morphological research compel us irresistibly to recognise the biogenetic law and its far-reaching consequences. These are, it is true, irreconcilable with the legends and doctrines of former days, that have been impressed on us by religious education. But without the biogenetic law, without the distinction between palingenesis and cenogenesis, and without the theory of evolution on which we base it, it is quite impossible to understand the facts of organic development; without them we cannot cast the faintest gleam of explanation over this marvellous field of phenomena. But when we recognise the causal correlation of ontogeny and phylogeny expressed in this law, the wonderful facts of embryology are susceptible of a very simple explanation; they are found to be the necessary mechanical effects of the evolution of the stem, determined by the laws of heredity and adaptation. The correlative action of these laws under the universal influence of the struggle for existence, or—as we may say in a word, with Darwin—“natural selection,” is entirely adequate to explain the whole process of embryology in the light of phylogeny. It is the chief merit of Darwin that he explained by his theory of selection the correlation of the laws of heredity and adaptation that Lamarck had recognised, and pointed out the true way to reach a causal interpretation of evolution.
The phenomenon that it is most imperative to recognise in this connection is the inheritance of functional variations. Jean Lamarck was the first to appreciate its fundamental importance in 1809, and we may therefore justly give the name of Lamarckism to the theory of descent he based on it. Hence the radical opponents of the latter have very properly directed their attacks chiefly against the former. One of the most distinguished and most narrow-minded of these opponents, Wilhelm His, affirms very positively that “characteristics acquired in the life of the individual are not inherited.”
The inheritance of acquired characters is denied, not only by thorough opponents of evolution, but even by scientists who admit it and have contributed a good deal to its establishment, especially Weismann, Galton, Ray Lankester, etc. Since 1884 the chief opponent has been August Weismann, who has rendered the greatest service in the development of Darwin’s theory of selection. In his work on The Continuity of the Germ-plasm, and in his recent excellent Lectures on the Theory of Descent (1902), he has with great success advanced the opinion that “only those characters can be transmitted to subsequent generations that were contained in rudimentary form in the embryo.” However, this germ-plasm theory, with its attempt to explain heredity, is merely a “provisional molecular hypothesis”; it is one of those metaphysical speculations that attribute the evolutionary phenomena exclusively to internal causes, and regard the influence of the environment as insignificant. Herbert Spencer, Theodor Eimer, Lester Ward, Hering, and Zehnder have pointed out the untenable consequences of this position. I have given my view of it in the tenth edition of the History of Creation (pp. 192, 203). I hold, with Lamarck and Darwin, that the hereditary transmission of acquired characters is one of the most important phenomena in biology, and is proved by thousands of morphological and physiological experiences. It is an indispensable foundation of the theory of evolution.
Of the many and weighty arguments for the truth of this conception of evolution I will for the moment merely point to the invaluable evidence of dysteleology, the science of rudimentary organs. We cannot insist too often or too strongly on the great morphological significance of these remarkable organs, which are completely useless from the physiological point of view. We find some of these useless parts, inherited from our lower vertebrate ancestors, in every system of organs in man and the higher Vertebrates. Thus we find at once on the skin a scanty and rudimentary coat of hair, only fully developed on the head, under the shoulders, and at a few other parts of the body. The short hairs on the greater part of the body are quite useless and devoid of physiological value; they are the last relic of the thicker hairy coat of our simian ancestors. The sensory apparatus presents a series of most remarkable rudimentary organs. We have seen that the whole of the shell of the external ear, with its cartilages, muscles, and skin, is in man a useless appendage, and has not the physiological importance that was formerly ascribed to it. It is the degenerate remainder of the pointed, freely moving, and more advanced mammal ear, the muscles of which we still have, but cannot work them. We found at the inner corner of our eye a small, curious, semi-lunar fold that is of no use whatever to us, and is only interesting as the last relic of the nictitating membrane, the third, inner eye-lid that had a distinct physiological purpose in the ancient sharks, and still has in many of the Amniotes.
The motor apparatus, in both the skeleton and muscular systems, provides a number of interesting dysteleological arguments. I need only recall the projecting tail of the human embryo, with its rudimentary caudal vertebræ and muscles; this is totally useless in man, but very interesting as the degenerate relic of the long tail of our simian ancestors. From these we have also inherited various bony processes and muscles, which were very useful to them in climbing trees, but are useless to us. At various points of the skin we have cutaneous muscles which we never use—remnants of a strongly-developed cutaneous muscle in our lower mammal ancestors. This “panniculus carnosus” had the function of contracting and creasing the skin to chase away the flies, as we see every day in the horse. Another relic in us of this large cutaneous muscle is the frontal muscle, by which we knit our forehead and raise our eye-brows; but there is another considerable relic of it, the large cutaneous muscle in the neck (platysma myoides), over which we have no voluntary control.
Not only in the systems of animal organs, but also in the vegetal apparatus, we find a number of rudimentary organs, many of which we have already noticed. In the alimentary apparatus there are the thymus-gland and the thyroid gland, the seat of goitre and the relic of a ciliated groove that the Tunicates and Acrania still have in the gill-pannier; there is also the vermiform appendix to the cæcum. In the vascular system we have a number of useless cords which represent relics of atrophied vessels that were once active as blood-canals—the ductus Botalli between the pulmonary artery and the aorta, the ductus venosus Arantii between the portal vein and the vena cava, and many others. The many rudimentary organs in the urinary and sexual apparatus are particularly interesting. These are generally developed in one sex and rudimentary in the other. Thus the spermaducts are formed from the Wolffian ducts in the male, whereas in the female we have merely rudimentary traces of them in Gaertner’s canals. On the other hand, in the female the oviducts and womb are developed from the Mullerian ducts, while in the male only the lowest ends of them remain as the “male womb” (vesicula prostatica). Again, the male has in his nipples and mammary glands the rudiments of organs that are usually active only in the female.
A careful anatomic study of the human frame would disclose to us numbers of other rudimentary organs, and these can only be explained on the theory of evolution. Robert Wiedersheim has collected a large number of them in his work on The Human Frame as a Witness to its Past. They are some of the weightiest proofs of the truth of the mechanical conception and the strongest disproofs of the teleological view. If, as the latter demands, man or any other organism had been designed and fitted for his life-purposes from the start and brought into being by a creative act, the existence of these rudimentary organs would be an insoluble enigma; it would be impossible to understand why the Creator had put this useless burden on his creatures to walk a path that is in itself by no means easy. But the theory of evolution gives the simplest possible explanation of them. It says: The rudimentary organs are parts of the body that have fallen into disuse in the course of centuries; they had definite functions in our animal ancestors, but have lost their physiological significance. On account of fresh adaptations they have become superfluous, but are transmitted from generation to generation by heredity, and gradually atrophy.
We have inherited not only these rudimentary parts, but all the organs of our body, from the mammals—proximately from the apes. The human body does not contain a single organ that has not been inherited from the apes. In fact, with the aid of our biogenetic law we can trace the origin of our various systems of organs much further, down to the lowest stages of our ancestry. We can say, for instance, that we have inherited the oldest organs of the body, the external skin and the internal coat of the alimentary system, from the Gastræads; the nervous and muscular systems from the Platodes; the vascular system, the body-cavity, and the blood from the Vermalia; the chorda and the branchial gut from the Prochordonia; the articulation of the body from the Acrania; the primitive skull and the higher sense-organs from the Cyclostomes; the limbs and jaws from the Selachii; the five-toed foot from the Amphibia; the palate from the Reptiles; the hairy coat, the mammary glands, and the external sexual organs from the Pro-mammals. When we formulated “the law of the ontogenetic connection of systematically related forms,” and determined the relative age of organs, we saw how it was possible to draw phylogenetic conclusions from the ontogenetic succession of systems of organs.
With the aid of this important law and of comparative anatomy we were also enabled to determine “man’s place in nature,” or, as we put it, assign to man his position in the classification of the animal kingdom. In recent zoological classification the animal world is divided into twelve stems or phyla, and these are broadly sub-divided into about sixty classes, and these classes into at least 300 orders. In his whole organisation man is most certainly, in the first place, a member of one of these stems, the vertebrate stem; secondly, a member of one particular class in this stem, the Mammals; and thirdly, of one particular order, the order of Primates. He has all the characteristics that distinguish the Vertebrates from the other eleven animal stems, the Mammals from the other sixty classes, and the Primates from the 300 other orders of the animal kingdom. We may turn and twist as we like, but we cannot get over this fact of anatomy and classification. Of late years this fact has given rise to a good deal of discussion, and especially of controversy as to the particular anatomic relationship of man to the apes. The most curious opinions have been advanced on this “ape-question,” or “pithecoid-theory.” It is as well, therefore, to go into it once more and distinguish the essential from the unessential. (Cf. pp. 261–5.)
We start from the undisputed fact that man is in any case—whether we accept or reject his special blood-relationship to the apes—a true mammal; in fact, a placental mammal. This fundamental fact can be proved so easily at any moment from comparative anatomy that it has been universally admitted since the separation of the Placentals from the lower mammals (Marsupials and Monotremes). But for every consistent subscriber to the theory of evolution it must follow at once that man descends from a common stem-form with all the other Placentals, the stem-ancestor of the Placentals, just as we must admit a common mesozoic ancestor of all the mammals. This is, however, to settle decisively the great and burning question of man’s place in nature, whether or no we go on to admit a nearer or more distant relationship to the apes. Whether man is or is not a member of the ape-order (or, if you prefer, the primate-order.) in the phylogenetic sense, in any case his direct blood-relationship to the rest of the mammals, and especially the Placentals, is established. It is possible that the affinities of the various orders of mammals to each other are different from what we hypothetically assume to-day. But, in any case, the common descent of man and all the other mammals from one stem-form is beyond question. This long-extinct Promammal was probably evolved from Proreptiles during the Triassic period, and must certainly be regarded as the monotreme and oviparous ancestor of all the mammals.
If we hold firmly to this fundamental and most important thesis, we shall see the “ape-question” in a very different light from that in which it is usually regarded. Little reflection is then needed to see that it is not nearly so important as it is said to be. The origin of the human race from a series of mammal ancestors, and the historic evolution of these from an earlier series of lower vertebrate ancestors, together with all the weighty conclusions that every thoughtful man deduces therefrom, remain untouched; so far as these are concerned, it is immaterial whether we regard true “apes” as our nearest ancestors or not. But as it has become the fashion to lay the chief stress in the whole question of man’s origin on the “descent from the apes,” I am compelled to return to it once more, and recall the facts of comparative anatomy and ontogeny that give a decisive answer to this “ape-question.”
The shortest way to attain our purpose is that followed by Huxley in 1863 in his able work, which I have already often quoted, Man’s Place in Nature—the way of comparative anatomy and ontogeny. We have to compare impartially all man’s organs with the same organs in the higher apes, and then to examine if the differences between the two are greater than the corresponding differences between the higher and the lower apes. The indubitable and incontestable result of this comparative-anatomical study, conducted with the greatest care and impartiality, was the pithecometra-principle, which we have called the Huxleian law in honour of its formulator—namely, that the differences in organisation between man and the most advanced apes we know are much slighter than the corresponding differences in organisation between the higher and lower apes. We may even give a more precise formula to this law, by excluding the Platyrrhines or American apes as distant relatives, and restricting the comparison to the narrower family-circle of the Catarrhines, the apes of the Old World. Within the limits of this small group of mammals we found the structural differences between the lower and higher catarrhine apes—for instance, the baboon and the gorilla—to be much greater than the differences between the anthropoid apes and man. If we now turn to ontogeny, and find, according to our “law of the ontogenetic connection of systematically related forms,” that the embryos of the anthropoid apes and man retain their resemblance for a longer time than the embryos of the highest and the lowest apes, we are forced, whether we like it or no, to recognise our descent from the order of apes. We can assuredly construct an approximate picture in the imagination of the form of our early Tertiary ancestors from the foregoing facts of comparative anatomy; however we may frame this in detail, it will be the picture of a true ape, and a distinct catarrhine ape. This has been shown so well by Huxley (1863) that the recent attacks of Klaatsch, Virchow, and other anthropologists, have completely failed (cf. pp.263–264). All the structural characters that distinguish the Catarrhines from the Platyrrhines are found in man. Hence in the genealogy of the mammals we must derive man immediately from the catarrhine group, and locate the origin of the human race in the Old World. Only the early root-form from which both descended was common to them.
It is, therefore, established beyond question for all impartial scientific inquiry that the human race comes directly from the apes of the Old World; but, at the same time, I repeat that this is not so important in connection with the main question of the origin of man as is commonly supposed. Even if we entirely ignore it, all that we have learned from the zoological facts of comparative anatomy and ontogeny as to the placental character of man remains untouched. These prove beyond all doubt the common descent of man and all the rest of the mammals. Further, the main question is not in the least affected if it is said: “It is true that man is a mammal; but he has diverged at the very root of the class from all the other mammals, and has no closer relationship to any living group of mammals.” The affinity is more or less close in any case, if we examine the relation of the mammal class to the sixty other classes of the animal world. Quite certainly the whole of the mammals, including man, have had a common origin; and it is equally certain that their common stem-forms were gradually evolved from a long series of lower Vertebrates.
The resistance to the theory of a descent from the apes is clearly due in most men to feeling rather than to reason. They shrink from the notion of such an origin just because they see in the ape organism a caricature of man, a distorted and unattractive image of themselves, because it hurts man’s æsthetic complacency and self-ennoblement. It is more flattering to think we have descended from some lofty and god-like being; and so, from the earliest times, human vanity has been pleased to believe in our origin from gods or demi-gods. The Church, with that sophistic reversal of ideas of which it is a master, has succeeded in representing this ridiculous piece of vanity as “Christian humility”; and the very men who reject with horror the notion of an animal origin, and count themselves “children of God,” love to prate of their “humble sense of servitude.” In most of the sermons that have poured out from pulpit and altar against the doctrine of evolution human vanity and conceit have been a conspicuous element; and, although we have inherited this very characteristic weakness from the apes, we must admit that we have developed it to a higher degree, which is entirely repudiated by sound and normal intelligence. We are greatly amused at all the childish follies that the ridiculous pride of ancestry has maintained from the Middle Ages to our own time; yet there is a large amount of this empty feeling in most men. Just as most people much prefer to trace their family back to some degenerate baron or some famous prince rather than to an unknown peasant, so most men would rather have as parent of the race a sinful and fallen Adam than an advancing, and vigorous ape. It is a matter of taste, and to that extent we cannot quarrel over these genealogical tendencies. Personally, the notion of ascent is more congenial to me than that of descent. It seems to me a finer thing to be the advanced offspring of a simian ancestor, that has developed progressively from the lower mammals in the struggle for life, than the degenerate descendant of a god-like being, made from a clod, and fallen for his sins, and an Eve created from one of his ribs. Speaking of the rib, I may add to what I have said about the development of the skeleton, that the number of ribs is just the same in man and woman. In both of them the ribs are formed from the middle germinal layer, and are, from the phylogenetic point of view, lower or ventral vertebral arches.
But it is said: “That is all very well, as far as the human body is concerned; on the facts quoted it is impossible to doubt that it has really and gradually been evolved from the long ancestral series of the Vertebrates. But it is quite another thing as regards man’s mind, or soul; this cannot possibly have been developed from the vertebrate-soul.”[35] Let us see if we cannot meet this grave stricture from the well-known facts of comparative anatomy, physiology, and embryology. It will be best to begin with a comparative study of the souls of various groups of Vertebrates. Here we find such an enormous variety of vertebrate souls that, at first sight, it seems quite impossible to trace them all to a common “Primitive Vertebrate.” Think of the tiny Amphioxus, with no real brain but a simple medullary tube, and its whole psychic life at the very lowest stage among the Vertebrates. The following group of the Cyclostomes are still very limited, though they have a brain. When we pass on to the fishes, we find their intelligence remaining at a very low level. We do not see any material advance in mental development until we go on to the Amphibia and Reptiles. There is still greater advance when we come to the Mammals, though even here the minds of the Monotremes and of the stupid Marsupials remain at a low stage. But when we rise from these to the Placentals we find within this one vast group such a number of important stages of differentiation and progress that the psychic differences between the least intelligent (such as the sloths and armadillos) and the most intelligent Placentals (such as the dogs and apes) are much greater than the psychic differences between the lowest Placentals and the Marsupials or Monotremes. Most certainly the differences are far greater than the differences in mental power between the dog, the ape, and man. Yet all these animals are genetically-related members of a single natural class.
[35] The English reader will recognise here the curious position of Dr. Wallace and of the late Dr. Mivart.—Translator.
We see this to a still more astonishing extent in the comparative psychology of another class of animals, that is especially interesting for many reasons—the insect class. It is well known that we find in many insects a degree of intelligence that is found in man alone among the Vertebrates. Everybody knows of the famous communities and states of bees and ants, and of the very remarkable social arrangements in them, such as we find among the more advanced races of men, but among no other group of animals. I need only mention the social organisation and government of the monarchic bees and the republican ants, and their division into different conditions—queen, drone-nobles, workers, educators, soldiers, etc. One of the most remarkable phenomena in this very interesting province is the cattle-keeping of the ants, which rear plant-lice as milch-cows and regularly extract their honeyed juice. Still more remarkable is the slave-holding of the large red ants, which steal the young of the small black ants and bring them up as slaves. It has long been known that these political and social arrangements of the ants are due to the deliberate cooperation of the countless citizens, and that they understand each other. A number of recent observers, especially Fritz Müller, Sir J. Lubbock (Lord Avebury), and August Forel, have put the astonishing degree of intelligence of these tiny Articulates beyond question.
Now, compare with these the mental life of many of the lower, especially the parasitic insects, as Darwin did. There is, for instance, the cochineal insect (Coccus), which, in its adult state, has a motionless, shield-shaped body, attached to the leaves of plants. Its feet are atrophied. Its snout is sunk in the tissue of the plants of which it absorbs the sap. The whole psychic life of these inert female parasites consists in the pleasure they experience from sucking the sap of the plant and in sexual intercourse with the males. It is the same with the maggot-like females of the fan-fly (Strepsitera), which spend their lives parasitically and immovably, without wings or feet, in the abdomen of wasps. There is no question here of higher psychic action. If we compare these sluggish parasites with the intelligent and active ants, we must admit that the psychic differences between them are much greater than the psychic differences between the lowest and highest mammals, between the Monotremes, Marsupials, and armadillos on the one hand, and the dog, ape, or man on the other. Yet all these insects belong to the same class of Articulates, just as all the mammals belong to one and the same class. And just as every consistent evolutionist must admit a common stem-form for all these insects, so he must also for all the mammals.
If we now turn from the comparative study of psychic life in different animals to the question of the organs of this function, we receive the answer that in all the higher animals they are always bound up with certain groups of cells, the ganglionic cells or neurona that compose the nervous system. All scientists without exception are agreed that the central nervous system is the organ of psychic life in the animal, and it is possible to prove this experimentally at any moment. When we partially or wholly destroy the central nervous system, we extinguish in the same proportion, partially or wholly, the “soul” or psychic activity of the animal. We have, therefore, to examine the features of the psychic organ in man. The reader already knows the incontestable answer to this question. Man’s psychic organ is, in structure and origin, just the same organ as in all the other Vertebrates. It originates in the shape of a simple medullary tube from the outer membrane of the embryo—the skin-sense layer. The simple cerebral vesicle that is formed by the expansion of the head-part of this medullary tube divides by transverse constrictions into five, and these pass through more or less the same stages of construction in the human embryo as in the rest of the mammals. As these are undoubtedly of a common origin, their brain and spinal cord must also have a common origin.
Physiology teaches us further, on the ground of observation and experiment, that the relation of the “soul” to its organ, the brain and spinal cord, is just the same in man as in the other mammals. The one cannot act at all without the other; it is just as much bound up with it as muscular movement is with the muscles. It can only develop in connection with it. If we are evolutionists at all, and grant the causal connection of ontogenesis and phylogenesis, we are forced to admit this thesis: The human soul or psyche, as a function of the medullary tube, has developed along with it; and just as brain and spinal cord now develop from the simple medullary tube in every human individual, so the human mind or the psychic life of the whole human race has been gradually evolved from the lower vertebrate soul. Just as to-day the intricate structure of the brain proceeds step by step from the same rudiment in every human individual—the same five cerebral vesicles—as in all the other Craniotes; so the human soul has been gradually developed in the course of millions of years from a long series of craniote-souls. Finally, just as to-day in every human embryo the various parts of the brain differentiate after the special type of the ape-brain, so the human psyche has proceeded historically from the ape-soul.
It is true that this Monistic conception is rejected with horror by most men, and the Dualistic idea, which denies the inseparable connection of brain and mind, and regards body and soul as two totally different things, is still popular. But how can we reconcile this view with the known facts of evolution? It meets with difficulties equally great and insuperable in embryology and in phylogeny. If we suppose with the majority of men that the soul is an independent entity, which has nothing to do with the body originally, but merely inhabits it for a time, and gives expression to its experiences through the brain just as the pianist does through his instrument, we must assign a point in human embryology at which the soul enters into the brain; and at death again we must assign a moment at which it abandons the body. As, further, each human individual has inherited certain personal features from each parent, we must suppose that in the act of conception pieces were detached from their souls and transferred to the embryo. A piece of the paternal soul goes with-the spermatozoon, and a piece of the mother’s soul remains in the ovum. At the moment of conception, when portions of the two nuclei of the copulating cells join together to form the nucleus of the stem-cell, the accompanying fragments of the immaterial souls must also be supposed to coalesce.
On this Dualistic view the phenomena of psychic development are totally incomprehensible. Everybody knows that the new-born child has no consciousness, no knowledge of itself and the surrounding world. Every parent who has impartially followed the mental development of his children will find it impossible to deny that it is a case of biological evolutionary processes. Just as all other functions of the body develop in connection with their organs, so the soul does in connection with the brain. This gradual unfolding of the soul of the child is, in fact, so wonderful and glorious a phenomenon that every mother or father who has eyes to observe is never tired of contemplating it. It is only our manuals of psychology that know nothing of this development; we are almost tempted to think sometimes that their authors can never have had children themselves. The human soul, as described in most of our psychological works, is merely the soul of a learned philosopher, who has read a good many books, but knows nothing of evolution, and never even reflects that his own soul has had a development.
When these Dualistic philosophers are consistent they must assign a moment in the phylogeny of the human soul at which it was first “introduced” into man’s vertebrate body. Hence, at the time when the human body was evolved from the anthropoid body of the ape (probably in the Tertiary period), a specific human psychic element—or, as people love to say, “a spark of divinity”—must have been suddenly infused or breathed into the anthropoid brain, and been associated with the ape-soul already present in it. I need not insist on the enormous theoretical difficulties of this idea. I will only point out that this “spark of divinity,” which is supposed to distinguish the soul of man from that of the other animals, must be itself capable of development, and has, as a matter of fact, progressively developed in the course of human history. As a rule, reason is taken to be this “spark of divinity,” and is supposed to be an exclusive possession of humanity. But comparative psychology shows us that it is quite impossible to set up this barrier between man and the brute. Either we take the word “reason” in the wider sense, and then it is found in the higher mammals (ape, dog, elephant, horse) just as well as in most men; or else in the narrower sense, and then it is lacking in most men just as much as in the majority of animals. On the whole, we may still say of man’s reason what Goethe’s Mephistopheles said:—
Life somewhat better might content him
But for the gleam of heavenly light that Thou hast given him.
He calls it reason; thence his power’s increased
To be still beastlier than any beast.
If, then, we must reject these popular and, in some respects, agreeable Dualistic theories as untenable, because inconsistent with the genetic facts, there remains only the opposite or Monistic conception, according to which the human soul is, like any other animal soul, a function of the central nervous system, and develops in inseparable connection therewith. We see this ontogenetically in every child. The biogenetic law compels us to affirm it phylogenetically. Just as in every human embryo the skin-sense layer gives rise to the medullary tube, from the anterior end of which the five cerebral vesicles of the Craniotes are developed, and from these the mammal brain (first with the characters of the lower, then with those of the higher mammals); and as the whole of this ontogenetic process is only a brief, hereditary reproduction of the same process in the phylogenesis of the Vertebrates; so the wonderful spiritual life of the human race through many thousands of years has been evolved step by step from the lowly psychic life of the lower Vertebrates, and the development of every child-soul is only a brief repetition of that long and complex phylogenetic process. From all these facts sound reason must conclude that the still prevalent belief in the immortality of the soul is an untenable superstition. I have shown its inconsistency with modern science in the eleventh chapter of The Riddle of the Universe.
Here it may also be well to point out the great importance of anthropogeny, in the light of the biogenetic law, for the purposes of philosophy. The speculative philosophers who take cognizance of these ontogenetic facts, and explain them (in accordance with the law) phylogenetically, will advance the great questions of philosophy far more than the most distinguished thinkers of all ages have yet succeeded in doing. Most certainly every clear and consistent thinker must derive from the facts of comparative anatomy and ontogeny we have adduced a number of suggestive ideas that cannot fail to have an influence on the progress of philosophy. Nor can it be doubted that the candid statement and impartial appreciation of these facts will lead to the decisive triumph of the philosophic tendency that we call “Monistic” or “Mechanical,” as opposed to the “Dualistic” or “Teleological,” on which most of the ancient, medieval, and modern systems of philosophy are based. The Monistic or Mechanical philosophy affirms that all the phenomena of human life and of the rest of nature are ruled by fixed and unalterable laws; that there is everywhere a necessary causal connection of phenomena; and that, therefore, the whole knowable universe is a harmonious unity, a monon. It says, further, that all phenomena are due solely to mechanical or efficient causes, not to final causes. It does not admit free-will in the ordinary sense of the word. In the light of the Monistic philosophy the phenomena that we are wont to regard as the freest and most independent, the expressions of the human will, are subject just as much to rigid laws as any other natural phenomenon. As a matter of fact, impartial and thorough examination of our “free” volitions shows that they are never really free, but always determined by antecedent factors that can be traced to either heredity or adaptation. We cannot, therefore, admit the conventional distinction between nature and spirit. There is spirit everywhere in nature, and we know of no spirit outside of nature. Hence, also, the common antithesis of natural science and mental or moral science is untenable. Every science, as such, is both natural and mental. That is a firm principle of Monism, which, on its religious side, we may also denominate Pantheism. Man is not above, but in, nature.
It is true that the opponents of evolution love to misrepresent the Monistic philosophy based on it as “Materialism,” and confuse the philosophic tendency of this name with a wholly unconnected and despicable moral materialism. Strictly speaking, it would be just as proper to call our system Spiritualism as Materialism. The real Materialistic philosophy affirms that the phenomena of life are, like all other phenomena, effects or products of matter. The opposite extreme, the Spiritualistic philosophy, says, on the contrary, that matter is a product of energy, and that all material forms are produced by free and independent forces. Thus, according to one-sided Materialism, the matter is antecedent to the living force; according to the equally one-sided view of the Spiritist, it is the reverse. Both views are Dualistic, and, in my opinion, both are false. For us the antithesis disappears in the Monistic philosophy, which knows neither matter without force nor force without matter. It is only necessary to reflect for some time over the question from the strictly scientific point of view to see that it is impossible to form a clear idea of either hypothesis. As Goethe said, “Matter can never exist or act without spirit, nor spirit without matter.”
The human “spirit” or “soul” is merely a force or form of energy, inseparably bound up with the material sub-stratum of the body. The thinking force of the mind is just as much connected with the structural elements of the brain as the motor force of the muscles with their structural elements. Our mental powers are functions of the brain as much as any other force is a function of a material body. We know of no matter that is devoid of force, and no forces that are not bound up with matter. When the forces enter into the phenomenon as movements we call them living or active forces; when they are in a state of rest or equilibrium we call them latent or potential. This applies equally to inorganic and organic bodies. The magnet that attracts iron filings, the powder that explodes, the steam that drives the locomotive, are living inorganics; they act by living force as much as the sensitive Mimosa does when it contracts its leaves at touch, or the venerable Amphioxus that buries itself in the sand of the sea, or man when he thinks. Only in the latter cases the combinations of the different forces that appear as “movement” in the phenomenon are much more intricate and difficult to analyse than in the former.
Our study has led us to the conclusion that in the whole evolution of man, in his embryology and in his phylogeny, there are no living forces at work other than those of the rest of organic and inorganic nature. All the forces that are operative in it could be reduced in the ultimate analysis to growth, the fundamental evolutionary function that brings about the forms of both the organic and the inorganic. But growth itself depends on the attraction and repulsion of homogeneous and heterogeneous particles. Seventy-five years ago Carl Ernst von Baer summed up the general result of his classic studies of animal development in the sentence: “The evolution of the individual is the history of the growth of individuality in every respect.” And if we go deeper to the root of this law of growth, we find that in the long run it can always be reduced to that attraction and repulsion of animated atoms which Empedocles called the “love and hatred” of the elements.
Thus the evolution of man is directed by the same “eternal, iron laws” as the development of any other body. These laws always lead us back to the same simple principles, the elementary principles of physics and chemistry. The various phenomena of nature only differ in the degree of complexity in which the different forces work together. Each single process of adaptation and heredity in the stem-history of our ancestors is in itself a very complex physiological phenomenon. Far more intricate are the processes of human embryology; in these are condensed and comprised thousands of the phylogenetic processes.
In my General Morphology, which appeared in 1866, I made the first attempt to apply the theory of evolution, as reformed by Darwin, to the whole province of biology, and especially to provide with its assistance a mechanical foundation for the science of organic forms. The intimate relations that exist between all parts of organic science, especially the direct causal nexus between the two sections of evolution—ontogeny and phylogeny—were explained in that work for the first time by transformism, and were interpreted philosophically in the light of the theory of descent. The anthropological part of the General Morphology (Book vii) contains the first attempt to determine the series of man’s ancestors (vol. ii, p. 428). However imperfect this attempt was, it provided a starting-point for further investigation. In the thirty-seven years that have since elapsed the biological horizon has been enormously widened; our empirical acquisitions in paleontology, comparative anatomy, and ontogeny have grown to an astonishing extent, thanks to the united efforts of a number of able workers and the employment of better methods. Many important biological questions that then appeared to be obscure enigmas seem to be entirely settled. Darwinism arose like the dawn of a new day of clear Monistic science after the dark night of mystic dogmatism, and we can say now, proudly and gladly, that there is daylight in our field of inquiry.
Philosophers and others, who are equally ignorant of the empirical sources of our evidence and the phylogenetic methods of utilising it, have even lately claimed that in the matter of constructing our genealogical tree nothing more has been done than the discovery of a “gallery of ancestors,” such as we find in the mansions of the nobility. This would be quite true if the genealogy given in the second part of this work were merely the juxtaposition of a series of animal forms, of which we gathered the genetic connection from their external physiognomic resemblances. As we have sufficiently proved already, it is for us a question of a totally different thing—of the morphological and historical proof of the phylogenetic connection of these ancestors on the basis of their identity in internal structure and embryonic development; and I think I have sufficiently shown in the first part of this work how far this is calculated to reveal to us their inner nature and its historical development. I see the essence of its significance precisely in the proof of historical connection. I am one of those scientists who believe in a real “natural history,” and who think as much of an historical knowledge of the past as of an exact investigation of the present. The incalculable value of the historical consciousness cannot be sufficiently emphasised at a time when historical research is ignored and neglected, and when an “exact” school, as dogmatic as it is narrow, would substitute for it physical experiments and mathematical formulæ. Historical knowledge cannot be replaced by any other branch of science.
It is clear that the prejudices that stand in the way of a general recognition of this “natural anthropogeny” are still very great; otherwise the long struggle of philosophic systems would have ended in favour of Monism. But we may confidently expect that a more general acquaintance with the genetic facts will gradually destroy these prejudices, and lead to the triumph of the natural conception of “man’s place in nature.” When we hear it said, in face of this expectation, that this would lead to retrogression in the intellectual and moral development of mankind, I cannot refrain from saying that, in my opinion, it will be just the reverse; that it will promote to an enormous extent the advance of the human mind. All progress in our knowledge of truth means an advance in the higher cultivation of the human intelligence; and all progress in its application to practical life implies a corresponding improvement of morality. The worst enemies of the human race—ignorance and superstition—can only be vanquished by truth and reason. In any case, I hope and desire to have convinced the reader of these chapters that the true scientific comprehension of the human frame can only be attained in the way that we recognise to be the sole sound and effective one in organic science generally—namely, the way of Evolution.
A
Abiogenesis, 26
Accipenser, 234
Abortive ova, 55
Achromatin, 42
Achromin, 42
Acœla, 221
Acoustic nerve, the, 289, 290
Acquired characters, inheritance of, 349
Acrania, the, 182
Acroganglion, the, 268, 275
Adam’s apple, the, 184
Adapida, 257
Adaptation, 3, 5, 27
After-birth, the, 167
Agassiz, L., 34
Age of life, 200
Alimentary canal, evolution of the, 13, 14, 133, 308–17
— — structure of the, 169, 308–10
Allantoic circulation, the, 171
Allantois, development of the, 166
Allmann, 20
Amblystoma, 243
Amitotic cleavage, 40
Ammoconida, 217
Ammolynthus, 217
Amnion, the, 115
— formation of the, 134, 244
Amniotic fluid, the, 134
Amœba, the, 47–9, 210
Amphibia, the, 239
Amphichœrus, 221
Amphigastrula, 80
Amphioxus, the, 105, 181–95
— circulation of the, 184
— cœlomation of the, 95
— embryology of the, 191–95
— structure of the, 183–88
Amphirhina, 230
Anamnia, the, 115
Anatomy, comparative, 208
Animalculists, 12
Animal layer, the, 16
Annelids, the, 142, 219
Annelid theory, the, 142
Anomodontia, 246
Ant, intelligence of the, 353
Anthropithecus, 174, 262
Anthropogeny, 1
Anthropoid apes, the, 166, 173, 262
Anthropology, 1, 35
Anthropozoic period, 203
Antimera, 107
Anura, 243
Anus, the, 317
Anus, formation of the, 139
Aorta, the, 327
— development of the, 170
Ape and man, 157, 164, 261, 307, 351
Ape-man, the, 263
Apes, the, 257–60
Aphanocapsa, 210
Aphanostomum, 221
Appendicaria, 197
Appendix vermiformis, the, 32
Aquatic life, early prevalence of, 235
Ararat, Mount, 24
Archenteron, 64, 74
Archeolithic age, 203
Archicaryon, 55
Archicrania, 230
Archigastrula, 65, 193
Archiprimas, 263
Arctopitheca, 261
Area, the germinative, 121
Aristotle, 9
Arm, structure of the, 306
Arrow-worm, the, 191
Arterial arches, the, 325–26
— cone, the, 324
Arteries, evolution of the, 170, 323–24
Articulates, the, 142, 219
— skeleton of the, 294
Articulation, 141–42
Aryo-Romanic languages, the, 203
Ascidia, the, 181, 188–90
— embryology of the, 196–98
Ascula, 217
Asexual reproduction, 51
Atlas, the, 247
Atrium, the, 183, 185
— (heart), the, 326
Auditory nerve, the, 289, 290
Auricles of the heart, 325
Autolemures, 257
Axolotl, the, 243
B
Bacteria, 38, 210
Baer, K. E. von, 15–17
Balanoglossus, 226
Balfour, F., 21
Batrachia, 241
Bdellostoma Stouti, 78
Bee, generation of the, 9
Beyschlag, W., on evolution, 50
Bilateral symmetry, 66
— — origin of, 221
Bimana, 258
Biogenetic law, the, 2, 21, 23, 179, 349
Biogeny, 2
Bionomy, 33
Bird, evolution of the, 245
— ovum of the, 44–6, 80–1
Bischoff, W., 17
Bladder, evolution of the, 244, 339
Blastæa, the, 206, 213
Blastocœl, the, 62, 74
Blastocrene, the, 99
Blastocystis, the, 62, 119, 120
Blastoderm, the, 62
Blastodermic vesicle, the, 119
Blastoporus, the, 64
Blastosphere, the, 62, 119
Blastula, the, 62, 74
— the mammal, 119
Blood, importance of the, 318
— recent experiments in mixture of, 172
— structure of the, 319
Blood-cells, the, 319
Blood-vessels, the, 318–25
— development of the, 168
— of the vertebrate, 110
— origin of the, 320–21
Boniface VIII, Bull of, 10
Bonnet, 13
Borneo nosed-ape, the, 164
Boveri, Theodor, 185
Brachytarsi, 257
Brain and mind, 278, 354–56
— evolution of the, 8, 275–80
— in the fish, 276
— in the lower animals, 275
— structure of the, 273–74
Branchial arches, evolution of the, 303
— cavity, the, 183, 189
— system, the, 110
Branchiotomes, 149
Breasts, the, 113
Bulbilla, 184
C
Calamichthys, 234
Calcolynthus, 217
Capillaries, the, 323
Caracoideum, the, 249
Carboniferous strata, 202
Carcharodon, 234
Cardiac cavity, the, 170
Cardiocœl, the, 328
Caryobasis, 38, 54
Caryokinesis, 42
Caryolymph, 38, 54
Caryolyses, 42
Caryon, 37
Caryoplasm, 37
Catallacta, 213
Catarrhinæ, the, 173, 261
Catastrophic theory, the, 24
Caudate cells, 53
Cell, life of the, 41–3
— nature of the, 36–7
— size of the, 38
Cell theory, the, 18, 36
Cenogenesis, 4
Cenogenetic structures, 4
Cenozoic period, the, 203
Central body, the, 38, 42
Central nervous system, the, 273
Centrolecithal ova, 68
Centrosoma, the, 38, 42
Ceratodus, the, 76, 237
Cerebellum, the, 274
Cerebral vesicles, evolution of the, 276
Cerebrum, the, 273
Cestracion Japonicus, 75, 79
Chætognatha, 94
Chick, importance of the, in embryology, 11, 16
Child, mind of the, 8, 355
Chimpanzee, the, 174, 262
Chiromys, 257
Chiroptera, 258
Chirotherium, 239
Chondylarthra, 257
Chorda, the, 17, 95, 107, 183
— evolution of the, 296
Chordæa, the, 97
Chordalemma, the, 296
Chordaria, 97
Chordula, the, 3, 96, 191
Choriata, the, 166
Chorion, the, 119
— development of the, 165–6
— frondosum, 255
— læve, 255
Choroid coat, the, 286
Chorology, 33
Chromacea, 209
Chromatin, 42
Chroococcacea, 210
Chroococcus, the, 210
Church, opposition of, to science in Middle Ages, 10
Chyle, 318
Chyle-vessels, 324
Cicatricula, the, 45, 81
Ciliated cells, 53, 193
Cinghalese gynecomast, 114
Circulation in the lancelet, 184
Circulatory system, evolution of the, 321–25
— — structure of the, 318
Classification, 103
— evolutionary value of, 33
Clitoris, the, 345
Cloaca, the, 249, 317
Cnidaria, 217
Coccyx, the, 295
Cochineal insect, the, 354
Cochlea, the, 289
Cœcilia, 241
Cœcum, the, 310, 317
Cœlenterata, 20, 91, 93, 104
Cœlenteria, 221
Cœloma, the, 21, 64, 91
Cœlomæa, the, 98
Cœlomaria, 21, 91, 104, 221
Cœlomation, 93–4
Cœlom-theory, the, 21, 93
Cœlomula, the, 98
Colon, the, 310, 317
Comparative anatomy, 31
Conception, nature of, 51
Conjunctiva, the, 286
Conocyema, 215
Convoluta, 221
Copelata, the, 197
Copulative organs, evolution of the, 344–45
Corium, the, 108, 268
Cornea, the, 286
Corpora cavernosa, the, 345, 346
Corpora quadrigemina, 274
Corpora striata, 274
Corpus callosum, the, 274
Corpus vitreum, the, 285
Corpuscles of the blood, 319
Craniology, 303
Craniota, the, 182, 229
Cranium, the, 299
Creation, 23–4
Cretaceous strata, 202
Crossopterygii, 234
Crustacea, the, 142, 219
Cryptocœla, 221
Cryptorchism, 114
Crystalline lens, the, 285
— — development of the, 287
Cutaneous glands, 268
Cuttlefish, embryology of the, 9
Cuvier, G., 17, 24
Cyanophycea, 209
Cyclostoma, the, 188, 230–32
— ova of the, 75
Cyemaria, 214
Cynopitheca, 262
Cynthia, 191, 196
Cytoblastus, the, 37
Cytodes, 40
Cytoplasm, 37, 38
Cytosoma, 37
Cytula, the, 54
D
Dalton, 15
Darwin, C., 2, 5, 23, 28–9
Darwin, E., 28
Darwinism, 5, 28
Decidua, the, 167
Deciduata, 255
Deduction, nature of, 208
Degeneration theory, the, 219
Dentition of the ape and man, 259
Depula, 62
Descent of Man, 30
Design in organisms, 33
Deutoplasm, 44
Devonian strata, 202
Diaphragm, the, 309
— evolution of the, 328
Dicyema, 215
Dicyemida, 215
Didelphia, 248
Digonopora, 223
Dinosauria, 202
Dipneumones, 238
Dipneusta, 235–38
— ova of the, 75
Dipnoa, 236
Directive bodies, 54
Discoblastic ova, 68
Discoplacenta, 255
Dissatyrus, 174
Dissection, medieval decrees against, 10
Dohrn, Anton, 219
Döllinger, 15
Dorsal furrow, the, 125
— shield, the, 123
— zone, the, 129
Dromatherium, 248
Dualism, 6
Dubois, Eugen, 263
Ductus Botalli, the, 350
Ductus venosus Arantii, 350
Duodenum, the, 309, 317
Duration of embryonic development, 199
— of man’s history, 199
Dysteleology, 32
— proofs of, 349
E
Ear, evolution of the, 288–92
— structure of the, 288
— uselessness of the external, 32
Ear-bones, the, 289
Earth, age of the, 200–201
Echidna hystrix, 249
Ectoblast, 20, 64
Ectoderm, the, 20, 64
Edentata, 250
Efficient causes, 6
Egg of the bird, 44–6, 81
— or the chick, priority of the, 211
Elasmobranchs, the, 79
Embryo, human, development of the, 158
Embryology, 2
— evolutionary value of, 34
Embryonic development, duration of, 199
— disk, the, 121–22
— spot, the, 125
Encephalon, the, 273
Endoblast, 20, 64
Endothelia, 321
Enterocœla, 93, 223
Enteropneusta, 226
Entoderm, the, 20, 64
Eocene strata, 203
Eopitheca, 259
Epiblast, 20, 64
Epidermis, the, 108, 268
Epididymis, the, 342
Epigastrula, 80
Epigenesis, 11, 13
Epiglottis, the, 309
Epiphysis, the, 108
Episoma, 129
Episomites, 130, 194
Epispadia, 346
Epithelia, 37
Epitheria, 243, 253
Epovarium, the, 342
Equilibrium, sense of, 291
Esthonychida, 257
Eustachian tube, the, 289
Eutheria, 253
Eve, 12
Evolution theory, the, 11, 208
— inductive nature of, 30
Eye, evolution of the, 285–88
— structure of the, 285
Eyelid, the third, 32
Eyelids, evolution of the, 288
F
Fabricius ab Aquapendente, 10
Face, embryonic development of the, 284
Fat glands in the skin, 269
Feathers, evolution of, 270
Fertilisation, 51
— place of, 119
Fin, evolution of the, 239, 304
Final causes, 6
Flagellate cells, 193
Floating bladder, the, 233, 241
— — evolution of the, 314
Fœtal circulation, 170–71
Food-yelk, the, 67,
Foot, evolution of the, 241, 304–6
— of the ape and man, 258–59
Fore brain, the, 278
Fore kidneys, the, 336, 337
Fossiliferous strata, list of, 201
Fossils, 180
— scarcity of, 208
Free will, 356
Friedenthal, experiments of, 172
Frog, the, 241–42
— ova of the, 71–2
Frontonia, 224
Function and structure, 7
Furcation of ova, 72
G
Gaertner’s duct, 341, 350
Ganglia, commencement of, 268
Ganglionic cell, the, 39
Ganoids, 233, 234
Gastræa, the, 3, 20, 206
— formation of the, 213
Gastræa theory, the, 20, 64, 69
Gastræads, 69, 214
Gastremaria, 214
Gastrocystis, the, 62, 119, 120
Gastrophysema, 215
Gastrotricha, 224
Gastrula, the, 3, 20, 62
Gastrulation, 62
Gegenbaur, Carl, 220
— on evolution, 32
— on the skull, 300–1
Gemmation, 331
General Morphology, 8, 29
Genesis, 23
Genital pore, the, 335
Geological evolution, length of, 200
— periods, 201
Geology, methods of, 180
— rise of, 24
Germ-plasm, theory of, 349
Germinal disk, 46, 81
— layers, the, 14, 16
— — scheme of the, 92
— spot, the, 44
— vesicle, the, 43, 54
Germinative area, the, 121
Giant gorilla, the, 176
Gibbon, the, 173, 262
Gill-clefts and arches, 110
— formation of the, 151–52, 303
Gill-crate, the, 183, 189
Gills, disappearance of the, 244
Glœocapsa, 210
Gnathostoma, 230, 232
Goethe as an evolutionist, 27, 299
Goitre, 110
Gonads, the, 111
— formation of the, 149–50
Gonidia, 334
Gonochorism, beginning of, 322
Gonoducts, 335
Gonotomes, 146, 149
Goodsir, 189
Gorilla, the, 174, 176, 262
Graafian follicles, the, 17, 119, 347
Gregarinæ, 211
Gullet-ganglion, the, 190
Gut, evolution of the, 310–17
Gyrini, 242
Gynecomastism, 114
H
Hag-fish, the, 188
Hair, evolution of the, 270
— on the human embryo and infant, 271
Hair, restriction of, by sexual selection, 271
Haliphysema, 215
Halisauria, 202
Haller, Albrecht, 12
Halosphæra viridis, 213
Hand, evolution of the, 250, 304–6
— of the ape and man, 258
Hapalidæ, 261
Harderian gland, the, 288
Hare-lip, 284
Harrison, Granville, 161
Hartmann, 262
Harvey, 10
Hatschek, 192
Hatteria, 243, 246
Head-cavity, the, 138
Head-plates, the, 149
Heart, development of the, 7, 10, 111, 151, 170, 322, 324–27
— of the ascidia, 190
— position of the, 327
Helmholtz, 207
Helminthes, 223
Hepatic gut, the, 109, 316
Heredity, nature of, 3, 5, 27, 56–7, 349
Hermaphrodism, 9, 23, 114, 218, 322, 346
Hertwig, 21
Hesperopitheca, 259
His, W., 19
Histogeny, 18, 19
History of Creation, 6, 30
Holoblastic ova, 67, 71, 77
Homœosaurus, 244, 246
Homology of the germinal layers, 20
Hoof, evolution of the, 270
Hunterian ligament, the, 344
Huxleian law, the, 171, 257, 262
Huxley, T. H., 7, 20, 29
Hydra, the, 69, 217
Hydrostatic apparatus in the fish, 315
Hylobates, 173, 262
Hylodes Martinicensis, 241
Hyoid bone, the, 299
Hypermastism, 113
Hyperthelism, 113
Hypoblast, 20, 64
Hypobranchial groove, the, 110, 184, 226, 316
Hypodermis, the, 268
Hypopsodina, 257
Hyposoma, the, 129
Hyposomites, 130, 194
Hypospadia, 346
I
Ichthydina, 224
Ichthyophis glutinosa, 80
Ictopsida, 257
Ileum, the, 310
Immortality, Aristotle on, 10
Immortality of the soul, 58
Impregnation-rise, the, 55
Indecidua, 255
Indo-Germanic languages, 203
Induction and deduction, 31, 208
Inheritance of acquired characters, 349
Insects, intelligence of, 353
Interamniotic cavity, the, 165
Intestines, the, 309, 316–17
Invagination, 62
Iris, the, 286
J
Jacchus, 261
Java, ape-man of, 263, 264
Jaws, evolution of the, 301
Jurassic strata, 202
K
Kant, dualism of, 25
Kelvin, Lord, on the origin of life, 207
Kidneys, the, 111
— formation of the, 150–51, 336–42
Klaatsch, 262
Kölliker, 21
Kowalevsky, 191
L
Labia, the, 346
Labyrinth, the, 290
Lachrymal glands, 269
Lamarck, J., 23, 25–7
— theories of, 26, 349
Lamprey, the, 230
— ova of the, 75
Lancelet, the, 60, 181–95
— description of the, 105
Languages, evolution of, 203
Lanugo of the embryo, 271
Larynx, the, 309
— evolution of the, 314
Latebra, the, 45
Lateral plates, the, 129
Laurentian strata, 201
Lecithoma, the, 117
Leg, evolution of the, 304
— structure of the, 306
Lemuravida, 257
Lemurogona, 257
Lemurs, the, 257
Lepidosiren, 257
Leucocytes, 319
Life, age of, 200
Limbs, evolution of the, 152, 239, 304
Limiting furrow, the, 133
Linin, 42
Liver, the, 309, 317
Long-nosed ape, the, 164
Love, importance of in nature, 332
Lungs, the, 110
— evolution of the, 241, 314–15
Lyell, Sir C., 24
Lymphatic vessels, the, 318
Lymph-cells, the, 319
M
Macrogonidion, 331
Macrospores, 331
Magosphæra planula, 213
Male womb, the, 344, 350
Mallochorion, the, 166
Mallotheria, 257
Malpighian capsules, 339, 341
Mammal, characters of the, 112
— gastrulation of the, 84
Mammals, unity of the, 247–48
Mammary glands, the, 113, 269
Man and the ape, relation of, 262, 351
— origin of, 29
Man’s Place in Nature, 7, 29, 351
Mantle, the, 189
Mantle-folds, the, 185
Marsupials, the, 250–52
— ova of the, 85
Materialism, 356
Mathematical method, the, 30
Mechanical causes, 6
— embryology, 8, 19, 22
Meckel’s cartilage, 304
Medulla capitis, the, 273
— oblongata, the, 274
— spinalis, the, 273
Medullary groove, the, 125
— tube, the, 107, 128
— — formation of the, 131, 133, 227, 267, 276
Mehnert, E., on the biogenetic law, 5
Meroblastic ova, 67, 71, 78
Merocytes, 68, 321
Mesentery, the, 98, 109, 310, 316
Mesocardium, the, 327
Mesoderm, the, 20, 64, 90, 93
Mesogastria, 215
Mesonephridia, the, 338
Mesonephros, the, 336
Mesorchium, the, 344
Mesovarium, the, 344
Mesozoic period, the, 202
Metogaster, the, 64
Metagastrula, the, 67
Metamerism, 142
Metanephridia, the, 341
Metanephros, the, 336
Metaplasm, 39
Metastoma, 64, 222
Metatheria, 248
Metazoa, 20, 62
Metovum, the, 81
Microgonidian, 331
Microspores, 331
Middle ear, the, 291
Migration, effect of, 33
Milk, secretion of the, 269
Mind, evolution of, 353–54
— in the lower animals, 353
Miocene strata, 203
Mitosis, 40, 41
Monera, 40, 206, 209
Monism, 6, 356
Monodelphia, 248
Monogonopora, 223
Monopneumones, 238
Monotremes, 118, 249
— ova of the, 84
Monoxenia Darwinii, 60
Morea, the, 212
Morphology, 2, 27
Morula, the, 62, 212
Motor-germinative layer, the, 19
Mouth, development of the, 124, 139
— structure of the, 308
Mucous layer, the, 16
Müllerian duct, the, 341
Muscle-layer, the, 16
Muscles, evolution of the, 307
— of the ear, rudimentary, 292
Myotomes, 108, 146
Myxinoides, the, 188, 230
N
Nails, evolution of the, 270
Nasal pits, 284
Natural philosophy, 25
— selection, 26, 28, 349
Navel, the, 117, 134
Necrolemurs, 257
Nectocystis, the, 314
Nemertina, 224–26
Nephroduct, evolution of the, 338–39
Nephrotomes, 149, 338
Nerve-cell, the, 39
Nerves, animals without, 267
Nervous system, evolution of the, 7, 267
Neurenteric canal, the, 127
Nictitating membrane, the, 32, 286, 288
Nose, the, in man and the ape, 164
— development of the, 282–85
— structure of the, 283
Notochorda, the, 107
Nuclein, 37
Nucleolinus, 44
Nucleolus, the, 38, 44, 54
Nucleus of the cell, 37
O
Œsophagus, the, 309, 316
Oken, 5, 27, 300
Oken’s bodies, 339
Oligocene strata, 203
Olynthus, 217
On the generation of animals, 9
Ontogeny, 2, 23
— defective evidence of, 208
Opaque area, the, 122
Opossum, the, 252
— ova of the, 85
Optic nerve, the, 287
Optic thalami, 274
— vesicles, 286
Orang, the, 174, 262
Ornithodelphia, 248
Ornithorhyncus, 85, 249
Ornithostoma, 249
Ossicles of the ear, 289
Otoliths, 289
Ova, number of, 347
— of the lancelet, 192
Ovaries, evolution of the, 333–34
Oviduct, origin of the, 335, 342
Ovolemma, the, 44
Ovulists, 12
Ovum, discovery of the, 16
— nature of the, 40
— size of the, 44
P
Pachylemurs, the, 257
Pacinian corpuscles, 282
Paleontology, 2
— evolutionary evidence of, 31
— incompleteness of, 208
— rise of, 24
Paleozoic age, the, 202
Palingenesis, 4
Palingenetic structures, 4
Palæhatteria, 244, 246
Panniculus carnosus, the, 350
Paradidymis, the, 342
Parietal zone, the, 129
Parthenogenesis, 9, 13
Pastrana, Miss Julia, 164
Pedimana, 252
Pellucid area, the, 122
Pelvic cavity, the, 138
Pemmatodiscus gastrulaceus, 215
Penis-bone, the, 346
Penis, varieties of the, 345
Peramelida, 254
Periblastic ova, 68
Peribranchial cavity, the, 185, 190
Pericardial cavity, the, 328
Perichorda, the, 108, 183
— formation of the, 136
Perigastrula, 89
Permian strata, 202
Petromyzontes, the, 188, 230
Phagocytes, 49, 320
Pharyngeal ganglion, the, 275
Pharynx, the, 309
Philology, comparison with, 203
Philosophie Zoologique, 25
Philosophy and evolution, 6
Phycochromacea, 209
Phylogeny, 2, 23
Physemaria, 214
Physiology, backwardness of, 7
Phytomonera, 209
Pineal eye, the, 108
Pinna, the, 291
Pithecanthropus, 263, 264
Pithecometra-principle, the, 171
Placenta, the, 166, 253–54
Placentals, the, 166
— characters of the, 253
— gastrulation of the, 86
Planocytes, 49, 320
Plant-louse, parthenogenesis of the, 13
Planula, the, 89
Plasma-products, 38, 39
Plasson, 40, 59
Plastids, 36, 40, 209
Plastidules, 59
Platodaria, 221
Platodes, the, 221
Platyrrhinæ, 261
Pleuracanthida, 234
Pleural ducts, 328
Pliocene strata, 203
Polar cells, 54
Polyspermism, 58
Preformation theory, the, 11
Primary period, the, 202
Primates, the, 157, 257–60
Primatoid, 263
Primitive groove, the, 69, 82, 124, 125
— gut, the, 20, 63, 214
— kidneys, the, 111, 337
— mouth, the, 20, 63
— segments, 143
— streak, the, 100, 122
— vertebræ, 144, 195, 206, 229
Primordial period, the, 201
Prochordata, 192
Prochordonia, the, 192, 218
Prochoriata, 253
Prochorion, the, 44, 119
Proctodæum, the, 345
Procytella primordialis, 210
Prodidelphia, 256
Progaster, the, 20, 63
Progonidia, 333
Promammalia, 247
Pronephridia, the, 151
Pronucleus femininus, 54
— masculinus, 54
Properistoma, 69
Prorenal canals of the lancelet, 186
— duct, the, 132, 139, 186
— — evolution of the, 338
Proselachii, 234
Prosimiæ, the, 257
Prospermaria, 333
Prospondylus, 105, 229
Prostoma, 20, 63, 222
Protamniotes, 243–44
Protamœba, 210
Proterosaurus, the, 202, 244
Protists, 36, 38
Protonephros, 111, 336
Protophyta, 210
Protoplasm, 37, 209
Protopterus, 238
Prototheria, 248
Protovertebræ, 142, 144
Protozoa, 20, 210
Provertebral cavity, the, 148
— plates, the, 136, 144
Pseudocœla, 93, 221
Pseudopodia, 48
Pseudova, 13
Psychic life, evolution of the, 8
Psychology, 8
Pterosauria, 202
Pylorus, the, 309
Q
Quadratum, the, 247
Quadrumana, 258
Quaternary period, 203
R
Rabbit, ova of the, 86–7
Radiates, the, 103
Rathke’s canals, 341
Rectum, the, 317
Regner de Graaf, 119
Renal system, evolution of the, 335–42
Reproduction, nature of, 330–31
Reptiles, 245–47
Respiratory organs, evolution of the, 314–15
— pore, the, 183, 189
Retina, the, 286
Rhabdocœla, 222
Rhodocytes, 319
Rhopalura, 215
Rhyncocephala, 243
Ribs, the, 295
— number of the, 353
Rudimentary ear-muscles, 292
— organs, 32
— — list of, 349–50
— toes, 306
S
Sacculus, the, 289
Sagitta, 65, 66, 191
— cœlomation of, 93
Salamander, the, 241
— ova of the, 74
Sandal-shape of embryo, 128–29
Satyrus, 174, 262
Sauromammalia, 246
Sauropsida, 245
Scatulation theory, the, 12
Schizomycetes, 210
Schleiden, M., 18, 36
Schwann, T., 18, 36
Sclerotic coat, the, 286
Sclerotomes, 108, 143, 148
Scrotum, the, 344
Scyllium, nose of the, 283
Sea-squirt, the, 181, 188–90
Secondary period, the, 202
Segmentation, 60, 141–42
Segmentation-cells, 54
Segmentation-sphere, the, 17
Selachii, 223
— skull of the, 301
Selection, theory of, 28
Selenka, 166, 168
Semnopitheci, 262
Sense-organs, evolution of the, 151, 280
— number of the, 281
— origin of the, 281
Sensory nerves, 279
Serocœlom, the, 165
Serous layer, the, 16
Sex-organs, early vertebrate form of the, 111
— evolution of the, 333
Sexual reproduction, simplest forms of, 331
— selection, 30, 271–72
Shark, the, 233
— nose of the, 283
— ova of the, 75
— placenta of the, 9
— skull of the, 301
Shoulder-blade, the, 306
Sickle-groove, the, 82, 121
Sieve-membrane, the, 167
Silurian strata, 202
Simiæ, the, 257–60
Siphonophoræ, embryology of the, 21
Skeleton, structure of the, 294
Skeleton-plate, the, 148
Skin, the, 151
— evolution of, 266–69
— function of the, 269
Skin-layer, the, 16
Skull, evolution of the, 149, 299–303
— structure of the, 299
— vertebral theory of the, 300
Smell, the sense of, 282
Soul, evolution of the, 353–56
— nature of the, 58, 356
— phylogeny of the, 8
— seat of the, 278
Sound, sensations of, 289–90
Sozobranchia, 242
Space, sense of, 291
Species, nature of the, 23, 34
Speech, evolution of, 264
Spermaducts, 335, 342
Spermaries, evolution of the, 333–34
Spermatozoon, the, 52–3
— discovery of the, 12, 53
Spinal cord, development of the, 8
— structure of the, 273
Spirema, the, 42
Spiritualism, 356
Spleen, the, 318
Spondyli, 142
Sponges, classification of the, 34
— ova of the, 49
Spontaneous generation, 26, 206
Stegocephala, 239
Stem-cell, the, 54
Stem-zone, the, 129
Stomach, evolution of the, 311–14, 316
— structure of the human, 309
Strata, thickness of, 200–201
Struggle for life, the, 28
Subcutis, the, 268
Sweat glands, 269
T
Tactile corpuscles, 268, 282
Tadpole, the, 242
Tail, evolution of the, 242–43
— rudimentary, in man, 159, 295, 350
Tailed men, 160–61
Taste, the sense of, 282
Teeth, evolution of the, 314
— of the ape and man, 259
Teleostei, 234
Telolecithal ova, 67, 68
Temperature, sense of, 282
Terrestrial life, beginning of, 235
Tertiary period, the, 203
Theoria generationis, the, 13
Theories, value of, 181
Theromorpha, 246
Third eyelid, the, 286, 288
Thyroid gland, the, 110, 184, 315
Time-variations in ontogeny, 5
Tissues, primary and secondary, 37
Toad, the, 241
Tocosauria, 246
Toes, number of the, 240
Tori genitales, the, 346
Touch, the sense of, 282
Tracheata, 142, 219
Tread, the, 45, 81
Tree-frog, the, 241
Triassic strata, 202
Triton tæniatus, 74
Troglodytes, 174
Tunicates, the, 189
Turbellaria, 222
Turbinated bones, the, 283
Tympanic cavity, the, 288
U
Umbilical, cord, the, 117
— vesicle, the, 138
Unicellular ancestor of all animals, 47
— animals, 38, 47
Urachus, the, 317, 341
Urinary system, evolution of the, 335–42
Urogenital ducts, 335
Uterus masculinus, the, 344, 350
Utriculus, the, 289
V
Vasa deferentia, 335
Vascular layer, the, 16, 168
— system, evolution of the, 321–25
— — structure of the, 318
Vegetative layer, the, 16
Veins, evolution of the, 323–24
Ventral pedicle, the, 166
Ventricles of the heart, 325
Vermalia, 220, 223
Vermiform appendage, the, 32, 310, 317
Vertebræ, 142, 294
Vertebræa, 105
Vertebral arch, the, 148, 295
— column, the, 144
— — evolution of the, 296
— — structure of the, 294
Vertebrates, character of the, 104–10
— descent of the, 219–20
Vertebration, 142
Vesico-umbilical ligament, the, 341
Vesicula prostatica, the, 344, 350
Villi of the chorion, 165
Virchow, R., 35
— on the ape-man, 303
— on the evolution of man, 264
Virgin-birth, 9, 13
Vitalism, 6
Vitelline duct, the, 138
Volvocina, 213
W
Wallace, A. R., 29
Water, organic importance of, 200
Water vessels, 336
Weismann’s theories, 349
Wolff, C. F., 13
Wolffian bodies, 339
Wolffian duct, the, 341
Womb, evolution of the, 342–43
Y
Yelk, the, 43, 45, 67
Yelk-sac, the, 117, 134
Z
Zona pellucida, the, 44
Zonoplacenta, 255
Zoomonera, 209
Zoophytes, 20, 64, 104
End of the Project Gutenberg EBook of The Evolution of Man, by Ernst Haeckel
*** END OF THIS PROJECT GUTENBERG EBOOK THE EVOLUTION OF MAN ***
***** This file should be named 8700-h.htm or 8700-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/8/7/0/8700/
Produced by Produced by Sue Asscher and Derek Thompson
Updated editions will replace the previous one--the old editions will
be renamed.
Creating the works from print editions not protected by U.S. copyright
law means that no one owns a United States copyright in these works,
so the Foundation (and you!) can copy and distribute it in the United
States without permission and without paying copyright
royalties. Special rules, set forth in the General Terms of Use part
of this license, apply to copying and distributing Project
Gutenberg-tm electronic works to protect the PROJECT GUTENBERG-tm
concept and trademark. Project Gutenberg is a registered trademark,
and may not be used if you charge for the eBooks, unless you receive
specific permission. If you do not charge anything for copies of this
eBook, complying with the rules is very easy. You may use this eBook
for nearly any purpose such as creation of derivative works, reports,
performances and research. They may be modified and printed and given
away--you may do practically ANYTHING in the United States with eBooks
not protected by U.S. copyright law. Redistribution is subject to the
trademark license, especially commercial redistribution.
START: FULL LICENSE
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full
Project Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project
Gutenberg-tm electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or
destroy all copies of Project Gutenberg-tm electronic works in your
possession. If you paid a fee for obtaining a copy of or access to a
Project Gutenberg-tm electronic work and you do not agree to be bound
by the terms of this agreement, you may obtain a refund from the
person or entity to whom you paid the fee as set forth in paragraph
1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this
agreement and help preserve free future access to Project Gutenberg-tm
electronic works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the
Foundation" or PGLAF), owns a compilation copyright in the collection
of Project Gutenberg-tm electronic works. Nearly all the individual
works in the collection are in the public domain in the United
States. If an individual work is unprotected by copyright law in the
United States and you are located in the United States, we do not
claim a right to prevent you from copying, distributing, performing,
displaying or creating derivative works based on the work as long as
all references to Project Gutenberg are removed. Of course, we hope
that you will support the Project Gutenberg-tm mission of promoting
free access to electronic works by freely sharing Project Gutenberg-tm
works in compliance with the terms of this agreement for keeping the
Project Gutenberg-tm name associated with the work. You can easily
comply with the terms of this agreement by keeping this work in the
same format with its attached full Project Gutenberg-tm License when
you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are
in a constant state of change. If you are outside the United States,
check the laws of your country in addition to the terms of this
agreement before downloading, copying, displaying, performing,
distributing or creating derivative works based on this work or any
other Project Gutenberg-tm work. The Foundation makes no
representations concerning the copyright status of any work in any
country outside the United States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other
immediate access to, the full Project Gutenberg-tm License must appear
prominently whenever any copy of a Project Gutenberg-tm work (any work
on which the phrase "Project Gutenberg" appears, or with which the
phrase "Project Gutenberg" is associated) is accessed, displayed,
performed, viewed, copied or distributed:
This eBook is for the use of anyone anywhere in the United States and
most other parts of the world at no cost and with almost no
restrictions whatsoever. You may copy it, give it away or re-use it
under the terms of the Project Gutenberg License included with this
eBook or online at www.gutenberg.org. If you are not located in the
United States, you'll have to check the laws of the country where you
are located before using this ebook.
1.E.2. If an individual Project Gutenberg-tm electronic work is
derived from texts not protected by U.S. copyright law (does not
contain a notice indicating that it is posted with permission of the
copyright holder), the work can be copied and distributed to anyone in
the United States without paying any fees or charges. If you are
redistributing or providing access to a work with the phrase "Project
Gutenberg" associated with or appearing on the work, you must comply
either with the requirements of paragraphs 1.E.1 through 1.E.7 or
obtain permission for the use of the work and the Project Gutenberg-tm
trademark as set forth in paragraphs 1.E.8 or 1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any
additional terms imposed by the copyright holder. Additional terms
will be linked to the Project Gutenberg-tm License for all works
posted with the permission of the copyright holder found at the
beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including
any word processing or hypertext form. However, if you provide access
to or distribute copies of a Project Gutenberg-tm work in a format
other than "Plain Vanilla ASCII" or other format used in the official
version posted on the official Project Gutenberg-tm web site
(www.gutenberg.org), you must, at no additional cost, fee or expense
to the user, provide a copy, a means of exporting a copy, or a means
of obtaining a copy upon request, of the work in its original "Plain
Vanilla ASCII" or other form. Any alternate format must include the
full Project Gutenberg-tm License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works
provided that
* You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is owed
to the owner of the Project Gutenberg-tm trademark, but he has
agreed to donate royalties under this paragraph to the Project
Gutenberg Literary Archive Foundation. Royalty payments must be paid
within 60 days following each date on which you prepare (or are
legally required to prepare) your periodic tax returns. Royalty
payments should be clearly marked as such and sent to the Project
Gutenberg Literary Archive Foundation at the address specified in
Section 4, "Information about donations to the Project Gutenberg
Literary Archive Foundation."
* You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or destroy all
copies of the works possessed in a physical medium and discontinue
all use of and all access to other copies of Project Gutenberg-tm
works.
* You provide, in accordance with paragraph 1.F.3, a full refund of
any money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days of
receipt of the work.
* You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project
Gutenberg-tm electronic work or group of works on different terms than
are set forth in this agreement, you must obtain permission in writing
from both the Project Gutenberg Literary Archive Foundation and The
Project Gutenberg Trademark LLC, the owner of the Project Gutenberg-tm
trademark. Contact the Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
works not protected by U.S. copyright law in creating the Project
Gutenberg-tm collection. Despite these efforts, Project Gutenberg-tm
electronic works, and the medium on which they may be stored, may
contain "Defects," such as, but not limited to, incomplete, inaccurate
or corrupt data, transcription errors, a copyright or other
intellectual property infringement, a defective or damaged disk or
other medium, a computer virus, or computer codes that damage or
cannot be read by your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium
with your written explanation. The person or entity that provided you
with the defective work may elect to provide a replacement copy in
lieu of a refund. If you received the work electronically, the person
or entity providing it to you may choose to give you a second
opportunity to receive the work electronically in lieu of a refund. If
the second copy is also defective, you may demand a refund in writing
without further opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO
OTHER WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT
LIMITED TO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of
damages. If any disclaimer or limitation set forth in this agreement
violates the law of the state applicable to this agreement, the
agreement shall be interpreted to make the maximum disclaimer or
limitation permitted by the applicable state law. The invalidity or
unenforceability of any provision of this agreement shall not void the
remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in
accordance with this agreement, and any volunteers associated with the
production, promotion and distribution of Project Gutenberg-tm
electronic works, harmless from all liability, costs and expenses,
including legal fees, that arise directly or indirectly from any of
the following which you do or cause to occur: (a) distribution of this
or any Project Gutenberg-tm work, (b) alteration, modification, or
additions or deletions to any Project Gutenberg-tm work, and (c) any
Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of
computers including obsolete, old, middle-aged and new computers. It
exists because of the efforts of hundreds of volunteers and donations
from people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future
generations. To learn more about the Project Gutenberg Literary
Archive Foundation and how your efforts and donations can help, see
Sections 3 and 4 and the Foundation information page at
www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg Literary
Archive Foundation are tax deductible to the full extent permitted by
U.S. federal laws and your state's laws.
The Foundation's principal office is in Fairbanks, Alaska, with the
mailing address: PO Box 750175, Fairbanks, AK 99775, but its
volunteers and employees are scattered throughout numerous
locations. Its business office is located at 809 North 1500 West, Salt
Lake City, UT 84116, (801) 596-1887. Email contact links and up to
date contact information can be found at the Foundation's web site and
official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To SEND
DONATIONS or determine the status of compliance for any particular
state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations. To
donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic works.
Professor Michael S. Hart was the originator of the Project
Gutenberg-tm concept of a library of electronic works that could be
freely shared with anyone. For forty years, he produced and
distributed Project Gutenberg-tm eBooks with only a loose network of
volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as not protected by copyright in
the U.S. unless a copyright notice is included. Thus, we do not
necessarily keep eBooks in compliance with any particular paper
edition.
Most people start at our Web site which has the main PG search
facility: www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.